Dr Katie Boog Explores Updated Guidance from the Faculty of Sexual & Reproductive Healthcare on Combined Hormonal Contraception, Including How to Assess Suitability and Discuss Risks, Benefits, and Tailored Regimens
| Read This Article to Learn More About: |
|---|
Find key points and implementation actions for STPs, ICSs, and clinical pharmacists in general practice at the end of this articlE |
The combined oral contraceptive pill (COCP) has been available free of charge on the NHS for more than 50 years. Over the last five decades, the formulations of COCP have changed, with oestrogen doses reducing, newer progestogens becoming available, and more recently the availability of 17beta-estradiol formulations as an alternative to ethinylestradiol. Combined hormonal contraception (CHC) is also available in the form of a combined transdermal patch (CTP) and combined vaginal ring (CVR).
Despite newer, more effective, and safer methods of contraception becoming available, CHC remains the most commonly used method of hormonal contraception in the UK.1 Women like CHC because it is familiar and easily accessible, provides a predictable bleeding pattern, and offers many non-contraceptive benefits.
Faculty of Sexual & Reproductive Healthcare Guidance
In January 2019, the Faculty of Sexual & Reproductive Healthcare (FSRH) updated its guideline on Combined hormonal contraception.2 The guideline provides evidence-based recommendations for the components of a CHC consultation, which are
- assessing suitability
- choosing CHC or an alternative contraceptive method
- choosing the type, formulation, and regimen of CHC
- giving information to support safety and effective use
- follow up and review.
The updated guidance has a novel format, providing a CHC consultation template with links to the relevant sections of the document to allow clinicians to easily navigate the guideline. In addition, a quick reference summary has been created to provide a succinct guide for busy clinicians.3
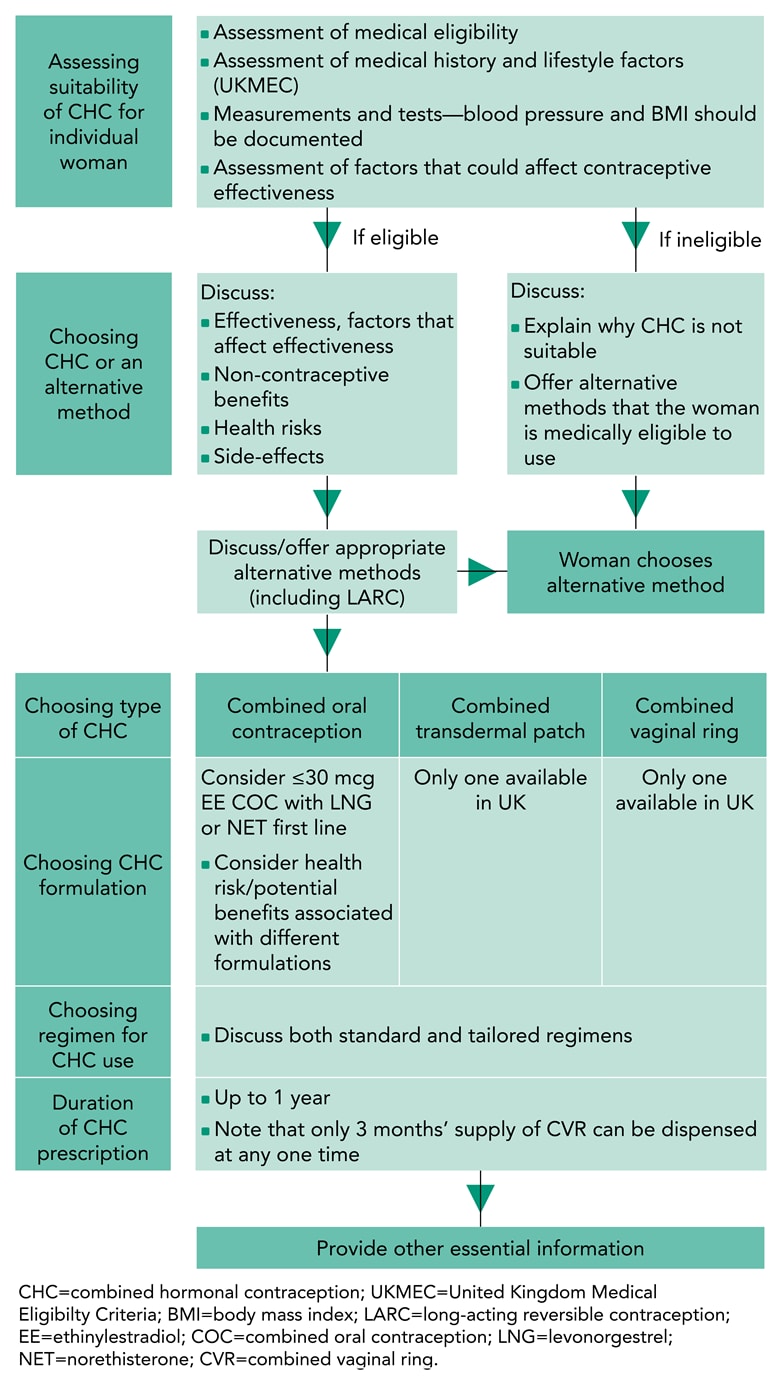
Assessing Suitability
For the majority of women, CHC is safe and the risk of a serious adverse event is very small.2 However, potential associated risks such as venous or arterial thromboembolism (VTE/ATE), breast cancer, and cervical cancer can be serious; progestogen-only and non-hormonal contraceptives may be safer alternatives.2,4
A thorough medical history needs to be taken to ensure CHC will be safe and effective for each individual woman. This history should include enquiring about any existing medical conditions; personal or family history of thromboembolic disease or breast cancer; lifestyle factors that may increase her risk of VTE or ATE (e.g. smoking); or factors that may reduce the efficacy of CHC, for example malabsorption or use of interacting medications.2 Blood pressure and body mass index (BMI) measurements should be taken.2
The guideline supports the use of suitable patient-completed questionnaires for assessing for any contraindications to CHC use. These have been shown to be useful and effective5–8 and may save the clinician time, as well as facilitating online or remote provision of CHC.
For women with existing health problems, the UK medical eligibility criteria for contraceptive use (UKMEC) should be consulted to assess the safety of CHC.4 There are no new contraindications to CHC use in the updated FSRH guideline on CHC,2 which remains in line with the 2016 UKMEC.4 The guideline is also aligned with the FSRH guidance on Contraception for women over 40 years, which explains that although CHC can be used until the age of 50, careful consideration needs to be given if it is to be used in women aged over 40 years because background risk of adverse events increases significantly with age.9
Efficacy of all CHC methods can be affected by concomitant use of hepatic enzyme-inducing drugs, both during treatment and for 28 days afterwards. In addition, efficacy of the COCP may be reduced by inadequate absorption (caused by diarrhoea, vomiting, drugs, or surgery that affect gut transit) and the CTP may be less effective for women who weigh 90 kg or more.2
Choosing CHC or an Alternative Method of Contraception
Clinicians should provide women with information about the risks and benefits of CHC, as well as alternative contraception options. Women should be advised that with perfect use, CHC will fail in the first year of use for 3 in 1000 women, but with typical use, the failure rate is estimated at 9%. In contrast, intrauterine and subdermal contraception have a failure rate under 1%.2
Where relevant, providers should inform women about the non-contraceptive benefits of CHC, such as improvement of acne; management of heavy menstrual bleeding, dysmenorrhoea, premenstrual syndrome, and polycystic ovary syndrome; and reduced risk of endometrial, ovarian, and colorectal cancer. For women in perimenopause, CHC use is also associated with a reduction in vasomotor symptoms and a protective effect on bone mineral density.2
Additionally, women should be advised of the small but potentially serious increased risks of VTE/ATE and breast and cervical cancer that are associated with CHC. They should be provided with information about the signs and symptoms of these, and when to seek medical review.2
Choosing Type and Formulation of CHC
If the clinician has ascertained that CHC will be safe for the woman and the woman has made an informed decision to choose CHC, she can then opt for the COCP, CVR, or CTP (see Figure 1). If a COCP is chosen, one containing ≤30 mcg ethinylestradiol in combination with levonorgestrel or norethisterone is suggested as a first-line option, as this will confer the lowest increase in risk of VTE (see Table 1). There is currently no evidence that COCPs containing 17beta-estradiol are safer than those containing ethinylestradiol.2 Non-oral methods may be chosen due to the woman’s personal preference or because of issues such as malabsorption.2
There is currently no evidence to suggest that unwanted side-effects are overall more or less common with any particular preparation of CHC.2 However, if a woman experiences side-effects with one formulation, she may find another formulation more suitable.
Faculty of Sexual & Reproductive Healthcare. Combined hormonal contraception. FSRH, January 2019 (updated February 2019). Available at: www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/
Table 1: European Medicines Agency Estimated Risk of Developing a Venous Thromboembolism in a Year According to Type of Combined Hormonal Contraception Used2,1
| Type of CHC Used | Risk of Developing a VTE in a Year (Incidence in 10,000 Women) |
|---|---|
| Women not using combined hormonal pill/patch/ring and not pregnant | ~2 |
| Women using CHC containing levonorgestrel,norethisterone or norgestimate | ~5–7 |
| Women using CHC containing etonogestrel or norelgestromin | ~6–12 |
| Women using CHC containing drospirenone,gestodene or desogestrel* | ~9–12 |
*Evidence suggests that co-cyprindiol is associated with similar VTE risk to combined oral contraceptive containing drospirenone, gestodene or desogestrel.11 CHC=combined hormonal contraception; VTE=venous thromboembolism Faculty of Sexual & Reproductive Healthcare. Combined hormonal contraception. FSRH, January 2019 (updated February 2019). Available at: www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/ | |
Choosing a Regimen
Note: Not all of the regimens discussed in this article currently (March 2019) have UK marketing authorisation. The prescriber should follow relevant professional guidance, taking full responsibility for all clinical decisions. Informed consent should be obtained and documented. See the General Medical Council’s guidance on Good practice in prescribing and managing medicines and devices12 for further information.
Traditionally, CHC has been taken for 21 days with a 7-day hormone-free interval (HFI). During the HFI, most women experience bleeding, which may be inconvenient or painful; they may also have unwanted symptoms such as headaches or mood changes due to the withdrawal of hormones. In addition, during the HFI there is follicular development,13–19 meaning that missing pills in the week before or after the HFI, or late replacement of a CVR or CTP, could extend the HFI sufficiently to result in ovulation and pregnancy.
There is no medical indication for women using CHC to have a withdrawal bleed, nor does it confirm that the woman is not pregnant.2 Therefore, women should be given information about both standard and tailored CHC and they may choose a tailored regimen, rather than the traditional 21/7 regimen (see Table 2). Current evidence suggests that tailored regimens are as safe and at least as effective as the traditional regimen.2
Table 2: Standard and Tailored Regimens for Use of Combined Hormonal Contraception2
| Type of Regimen | Period of CHC Use | HFI (Days) | |
|---|---|---|---|
| Standard use | 21 days (21 active pills or 1 ring, or 3 patches) | 7 | |
| Tailored use | Shortened hormone-free interval (HFI) | 21 days (21 active pills or 1 ring, or 3 patches) | 4 |
| Extended use (tricycling) | 9 weeks (3 x 21 active pills or 3 rings, or 9 patches used consecutively) | 4 or 7 | |
| Flexible extended use | Continuous use (≥21 days) of active pills, patches, or rings until breakthrough bleeding occurs for 3–4 days | 4 | |
| Continuous use | Continuous use of active pills, patches, or rings | None | |
CHC=combined hormonal contraception; HFI=hormone-free interval Faculty of Sexual & Reproductive Healthcare. Combined hormonal contraception. FSRH, January 2019 (updated February 2019). Available at: www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/ | |||
Tailored Regimens
Tricycling, continuous, and flexible extended regimens are suitable for women using a monophasic COCP, CVR, or CTP, but not a multiphasic COCP; multiphasic COC should not be used in tailored regimens.2
Women should have access to clear digital or written information about tailored CHC. As most patient information leaflets only provide information on the traditional regimen, the FSRH is working with the Family Planning Association to create patient resources for the tailored regimens.
Tailored regimens can:2
- shorten the HFI and/or
- reduce the frequency of the HFI and/or
- abolish the HFI.
With a shorter HFI, the woman still takes 21 days of active CHC but this is followed by a 4-day HFI instead of a 7-day HFI. This may reduce unwanted symptoms during the HFI and could theoretically mean that missing pills in the week before or after the HFI is less risky.
With the tricycling regimen, the woman takes 63 days of CHC (three packets of monophasic COCPs, CTPs, or CVRs) and then has a 4- or 7-day HFI. This reduces the frequency of HFI from 12 per year to just four.
As there is no medical indication for an HFI, it is possible for the woman to take CHC continuously, abolishing the HFI and any associated symptoms altogether. However, this may cause unpredictable bleeding which may or may not be acceptable to the woman.
An alternative to the continuous approach is the flexible extended regimen. The woman takes at least 21 days of active CHC and if she had no bleeding, she continues to take it. Once she has bleeding or spotting for 4 days in a row, she stops for a 4-day HFI. She then restarts CHC, taking it for at least 21 days and continuing until she has bleeding or spotting for 4 days in a row, at which point she has a 4-day HFI and then restarts, continuing this cycle.
Additional Recommendations
CHC Use During Travel or at High Altitudes
The guidance recommends that women should be advised to reduce periods of immobility when travelling; if they are trekking at high altitudes (above 4500 metres) for >7 days, they should switch to a safer, alternative method of contraception due to increased risk of thrombosis.2
CHC Use and Surgery/Immobility
Women who require major surgery or anticipate any periods of prolonged immobility should stop CHC at least 4 weeks beforehand and commence on an alternative, safer method of contraception.2
Missed Pills
The FSRH is currently working on an international consensus on missed pill guidance. In the meantime, interim guidance has been published on the FSRH website, separate from the CHC guideline.20 At present, this remains unchanged from previous guidance, except that additional precautions are now recommended if the HFI is extended beyond 7 days.20
Follow Up and Review
The guideline recommends that for women who plan to use the method for 12 months or more, a 12-month prescription of CHC can be given at both initiation and follow up. The small potential for waste if the woman chooses to discontinue the method is considered to be outweighed by the benefit of reduced follow ups and reduced clinician time. Removing restrictions on supply could prevent unwanted discontinuation that can lead to unplanned pregnancy. Some women (e.g. women with significant, relevant medical problems) may benefit from an earlier review. All women should have an annual assessment to review suitability, adherence, and satisfaction with the method and to obtain blood pressure and BMI measurements.2
Women should be advised when to seek earlier medical review: symptoms of VTE/ATE, breast changes, new onset of or changes to previous migraine, persistent unscheduled bleeding, or new medical diagnosis/medication.2
Further Information and Support
To support busy clinicians, a quick reference summary has been created as an FSRH members’ benefit and can be found in the standards and guidance section of the FSRH website.3 In addition, FSRH members can also access the CHC webinar, to help support them to understand and implement the new guidance. Patient resources for tailored regimens and further guidance on incorrect CHC use are expected to be available later in 2019.
Dr Katie Boog
Senior Registrar in Community Sexual and Reproductive Health Nottingham Integrated Sexual Health Service, Nottingham University Hospitals Trust
| Key Points |
|---|
CHC=combined hormonal contraception; BMI=body mass index |
| Implementation Actions for STPs and ICSs |
|---|
Written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved with implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system; FSRH=Faculty of Sexual & Reproductive Healthcare; CHC=combined hormonal contraception; LARC=long-acting reversible contraception; FAQs=frequently asked questions |
| Implementation Actions for Clinical Pharmacists in General Practice |
|---|
Written by Gupinder Syan, Training and Clinical Outcomes Manager, Soar Beyond Ltd The following implementation actions are designed to support clinical pharmacists in general practice with implementing the guidance at a practice level.
COCP=combined oral contraceptive pill; HCP=healthcare professional; FSRH=Faculty of Sexual & Reproductive Healthcare; CHC=combined hormonal contraception; LARC=long-acting reversible contraception; VTE=venous thromboembolism; ATE=arterial thromboembolism. |

