Dr Richard Simcock and Dr Nicola Harker Offer 10 Top Tips on Early Breast Cancer Diagnosis and Treatment, Based on Recent Recommendations and Research
| Read This Article to Learn More About: |
|---|
|
Breast cancer is diagnosed in approximately 150 women per day in the UK (over 55,000 invasive cases in 2015). Despite improvements in survival rates, approximately 31 women die of the disease each day (11,400 per year from 2014 to 2016). The incidence increases with age such that 1 in 8 UK women will be diagnosed with the cancer in their lifetime; 25% of cases will occur in women aged over 75.1 The incidence is slowly rising so that rates are 4% higher now than they were a decade ago but early diagnosis, screening, and progressive improvements in treatment mean that mortality rates are 38% lower than they were in the 1970s.1
Diagnosis of early‑stage disease proceeding to surgery remains the most important aspect of successful treatment. Promoting breast awareness (e.g. at oral contraceptive pill (OCP), hormone replacement therapy (HRT), or cervical smear appointments, and encouraging attendance at NHS screening appointments, usually help early diagnosis.
The increasing and longer use of adjuvant therapies (chemotherapy, radiotherapy, hormone therapy, and now also bisphosphonates) has impacted significantly on survival rates but creates treatment burden for patients and issues in primary care. This article will explore the key points and considerations for primary care practitioners when managing patients with breast cancer.
Not all of the treatments discussed in this article currently (September 2018) have UK marketing authorisation for the indications mentioned. The prescriber should follow relevant professional guidance, taking full responsibility for all clinical decisions. Informed consent should be obtained and documented. See the General Medical Council’s guidance on Good practice in prescribing and managing medicines and devices2 for further information.
1. Know About Predictive Tools
It is important that GPs know about the available tools that can be used to predict treatment outcomes. Adjuvant chemotherapy is a complex treatment with multiple toxicities including alopecia, fatigue, nausea, and impact on fertility. Use of chemotherapy should be proportionate to risk of disease recurrence or death. Decision tools are now available to guide consultations to help patients understand their risk of breast cancer death and the absolute benefits of chemotherapy. NICE recommends the use of the Predict tool, which uses UK population data. The tool produces helpful icon arrays and illustrations (Figure 1).3,4
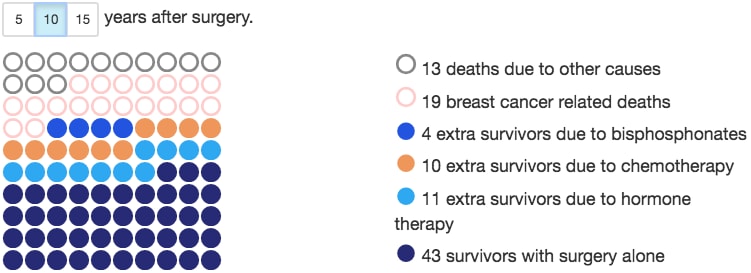
An example output from the Predict tool, predicting 10-year survival rates of a 65-year-old patient with breast cancer that is ER-positive, HER2-negative, has a Ki-67 ≤10%, and a grade 3, 35 mm tumour with two positive nodes detected by symptoms. This assumes treatment with third generation chemotherapy.
Eastern Cancer Registry and Information Centre and Cambridge University. Predict—breast cancer. Public Health England and Cambridge University. www.predict.nhs.uk/predict_v2.1/ (accessed 28 August 2018)
2. Know About Genomic Profiling
Genomic profiling of the patient’s breast tumour gives extra information on prognosis. In a recent study, the Oncotype DX® test was shown to predict a patient’s individual benefit from chemotherapy.5 Oncotype DX® is approved by NICE.6,7 Patients may have an extra wait for this test result (the test is performed on a section of the removed tumour which is then sent to a USA-based lab). Turnaround for UK patients is around 2–3 weeks but it may help them safely avoid chemotherapy.
3. Know When to Refer for Genetic Testing
As well as genomic profiling of the tumour, the rates of genetic testing of patients have also increased. The BRCA breast cancer predisposition gene may be present without a strong family history of breast cancer (particularly if the gene has been inherited and passed on by male ancestors). Thresholds for testing have reduced over time and all women under the age of 50 with a triple negative cancer (lacking receptors for oestrogen, progesterone, or HER2) should be tested.3 The genetics team at Guy’s and St Thomas’ Hospitals has produced a useful referral guidelines app called ‘Cancer Genetics’.
4. Discuss the Risks and Benefits of Radiotherapy
Radiotherapy is recommended standard therapy for most women who have had breast-conserving surgery and for some patients after mastectomy. The absolute benefits of radiotherapy can be very small and new NICE guidance encourages a discussion of risk, particularly in women aged over 70 with small cancers where radiotherapy might reasonably be avoided.3
When treatment is given it may be possible to deliver radiotherapy to only part of the breast to reduce discomfort, erythema, and longer-term fibrosis and breast lymphoedema.
NICE recommends a standard treatment schedule of 15 fractions3 (3 weeks) but UK trials of using only five sessions of treatment are currently in long-term follow up.
5. Update your Knowledge of Anti-oestrogen Therapies
For women with oestrogen-receptor (ER)-positive breast cancer (around 65–80% of the total9), treatment with an anti-oestrogen is a key component of adjuvant therapy. Endocrine therapies prevent recurrent disease but may also prevent new primary disease developing in either breast. Aromatase inhibitors (anastrozole, letrozole, and exemestane) may only be used in postmenopausal women while tamoxifen can be used in both premenopausal and postmenopausal women. Fulvestrant (a selective oestrogen receptor degrader) is not licensed for adjuvant therapy. Fulvestrant can be helpful in advanced disease but has not been approved by NICE on the basis of cost.10,11 Some cancer centres in the UK continue to have some access to the drug via hospital funding.
For most patients who are tolerating endocrine therapy, then extended adjuvant therapy (longer than 5 years) is now recommended3—usually to 10 years. The benefits of extending therapy to 10 years need to be balanced against toxicities (see below). Sequencing therapy (taking tamoxifen followed by an aromatase inhibitor or vice versa) appears to be a more effective prevention strategy than remaining on a single drug.12
NICE has outlined the effects of extending endocrine therapy (see Table 1).3
Table 1: Effects of Extended Endocrine Therapy3
| Extended Tamoxifen Therapy (After an Initial 5 years of Tamoxifen Therapy) | Extended Endocrine Therapy With an Aromatase Inhibitor (After 5 Years of Tamoxifen Therapy) | |
|---|---|---|
| Definition | Continuing to take tamoxifen after 5 years of tamoxifen therapy. | Switching to an aromatase inhibitor after 5 years of tamoxifen therapy. |
| Who can take this therapy | Premenopausal or postmenopausal women with ER‑positive invasive breast cancer. | Postmenopausal women with ER‑positive invasive breast cancer. |
Effect on breast cancer recurrence NOTE: The benefit for an individual person will depend on the risk of their cancer returning. For people with a low risk of recurrence, the benefits may not outweigh the risks or side-effects. Medium or high risk may include people who have lymph node‑positive breast cancer, with tumours that are T2 or greater and higher grade. Low risk may include people with lymph node‑negative breast cancer, with smaller or lower‑grade tumours. | Lower rates of breast cancer recurrence compared with 5 years of tamoxifen therapy. | Lower rates of breast cancer recurrence compared with 5 years of tamoxifen therapy. In postmenopausal women, switching to an aromatase inhibitor may be more effective at reducing recurrence than continuing with tamoxifen. |
Side-effects NOTE: These are common side-effects experienced during additional years taking endocrine therapy. Most effects are reversible when tablets are stopped. | Side-effects of endocrine therapy will continue for additional years (for example, menopausal symptoms such as hot flushes). With extended use of tamoxifen: increased risk of thrombosis and endometrial cancer, and possibly bone density loss in premenopausal women. | Side-effects of endocrine therapy will continue for additional years (for example, menopausal symptoms such as hot flushes). With extended use of aromatase inhibitors: bone density loss, and joint and muscle pain. |
| Fertility and family planning | Effects on fertility and family planning will continue for additional years as women should not become pregnant while taking tamoxifen, or within 2 months of stopping, because it may have adverse effects on the baby. | Not applicable as postmenopausal women only. |
| Reproduced from © NICE 2018 Early and locally advanced breast cancer: diagnosis and management. Available from www.nice.org.uk/ng101 All rights reserved. Subject to notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this publication. See www.nice.org.uk/re-usi ng-our-content/uk-open-content-licence for further details. | ||
6. Know About Menopause-inducing Therapies
In two large clinical trials (SOFT and TEXT), premenopausal women with ER-positive breast cancer had better breast cancer outcomes if they were rendered postmenopausal (with luteinising hormone-releasing hormone [LHRH] agonists like goserelin or triptorelin) and received an aromatase inhibitor in preference to tamoxifen.13,14 The benefits are greater in women with high-risk disease and particularly those under the age of 35 years. The effect of a medical menopause in these younger women, however, can be profound, with quite significant menopausal toxicities.
If a patient is well established on LHRH agonists as a medical menopause, it is safe and appropriate to prescribe a 3-monthly depot preparation in preference to less convenient monthly dosing.
7. Ask About Side-effects of Endocrine Therapies
Side-effects of endocrine therapies are significant and affect patients’ ability to stay on therapy. Up to 30% of patients may decide to stop taking aromatase inhibitors, half of these due to arthralgia.15 The arthralgia caused by aromatase inhibitors can be effectively and safely treated by acupuncture as demonstrated in a large, well‑conducted, double‑controlled trial, though it should be noted that the magnitude of improvement was of uncertain clinical importance.16 Macmillan suggests that keeping physically active, maintaining a healthy weight, and analgesia may also help with some side-effects.17
Vaginal dryness and atrophy are common complications of aromatase inhibitors. Remember to ask about dyspareunia as patients may not volunteer this information. Vulval discomfort and dryness may be problematic even if the patient is not sexually active. Water-based lubricants are useful for sex, and oil-based lubricants may be helpful for daily dryness. Some patients will need local oestrogen prescribing to relieve symptoms. Although studies do show a rise in systemic oestradiol levels with use of vaginal oestrogen, it has not been demonstrated to be unsafe and clear benefits to sexual health and libido are seen.18
The American College of Obstetricians and Gynecologists has supported the use of vaginal oestrogens in women with ER-positive breast cancer (although there is no similar UK guidance).19
Menopausal hot flushes are an exceptionally common side-effect. There are useful pharmacological and non-pharmacological measures which may help as well as some useful lifestyle advice (see Figure 2).
Updated NICE guidance continues to warn against systemic hormone replacement therapy (HRT) in women with ER-positive breast cancer but accepts there may be exceptional circumstances where menopausal symptoms are severe enough to justify HRT (after discussion of risks; a history of breast cancer is a relative contraindication to HRT).3
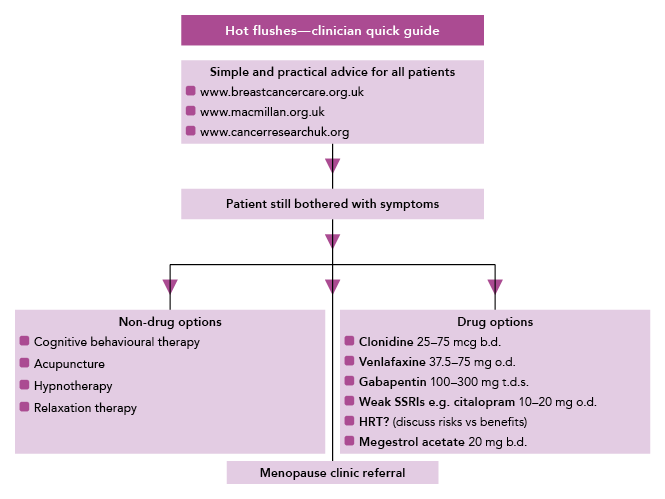
An algorithm exploring the methods of managing hot flushes in patients with breast cancer resulting from chemotherapy‑induced early menopause, endocrine therapy, or a combination of both. For many patients hot flushes can be managed with simple practical advice, such as wearing natural fabrics, using a handheld fan, and avoiding possible triggers (e.g. coffee, alcohol). Pharmacological treatment with paroxetine and fluoxetine should be avoided in patients receiving tamoxifen as these treatments can interact. Previous breast cancer is a relative contraindication to HRT. The use of megestrol acetate 20 mg has been shown to reduce the severity of hot flushes but its use is controversial and not currently recommended by NICE. Clinical studies to evaluate the safety of megestrol acetate are ongoing.
SSRI=selective serotonin reuptake inhibitors; HRT=hormone replacement therapy
Adapted from: National Cancer Research Institute breast cancer clinical studies group—symptom management subgroup. Hot flushes—clinician quick guide. Reproduced with permission.
8. Monitor for Treatment-induced Bone Loss
Long-term use of aromatase inhibitors can accelerate postmenopausal bone loss and all trials of the drugs showed small increases in osteoporotic fracture compared with controls.20 A DEXA scan is recommended at the outset of therapy.3 A UK expert group has produced algorithms for the management of bone health for patients with breast cancer treatment induced bone loss, which recommend early use of a bisphosphonate.21 As more breast cancer patients move to risk-stratified self-care, it is important to negotiate local protocols and responsibilities between primary and secondary care for arranging and reviewing these tests.
9. Know the Benefits of Certain Bisphosphonates
As well as protecting the bones of patients from the osteoporotic effects of anti‑oestrogens, bisphosphonates have also been shown to be useful as an adjuvant therapy in their own right. Bisphosphonates have been used for many years in women with metastatic breast cancer in bone (and men with prostate cancer) to prevent and reduce skeletal-related events (fracture, pain, or malignant spinal cord compression). A recent large meta-analysis has now also demonstrated that bisphosphonates used as adjuvant therapy improve overall survival for postmenopausal women and NICE has approved their use in these women.3,22 The bisphosphonates used in the study were mainly zoledronic acid (intravenous) or sodium clodronate and at doses much higher than those typically used to treat osteoporosis. NICE has specifically recommended these two drugs, alongside a discussion of their associated risks.3 There are no data at all on the efficacy of alendronate and it is not recommended in this indication.
A trial using denosumab as an adjuvant treatment for breast cancer has not shown improvements in survival although, curiously, the drug appears to be superior to bisphosphonates in preventing bone problems in the treatment of metastatic breast cancer.23
10. Be Aware of Cardiotoxicity
Adjuvant therapy for breast cancer can be cardiotoxic. Anthracyclines used in chemotherapy may cause left ventricular failure in a dose‑dependent way, which sometimes manifests many years later. The use of anthracyclines is slowly declining partly due to this issue.
The monoclonal antibody trastuzumab is routinely prescribed as adjuvant therapy for women with HER2-positive breast cancer (approximately 20% of all cases)9 and will cause a measurable reduction in left ventricular ejection fraction in 8% of patients during the standard 12 months of therapy.24 NICE recommends that regular cardiac function assessments are organised by the prescribing team in hospital; these tests are now part of routine oncological care for patients with HER2-positive disease and coordinated by the oncology team.3 A large UK trial (the PERSEPHONE study) suggests that 6 months of therapy with trastuzumab may be adequate, with a 50% reduction in heart damage (and inconvenience to the patient and cost to the NHS).24 Trastuzumab-induced left ventricular failure (LVF) is treated with an ACE inhibitor.
Finally, patients with left‑sided breast cancer who received radiotherapy in previous decades may have received inadvertent cardiac irradiation, which predisposes them to increased cardiac events in later life and should be considered in the history of patients presenting with cardiac problems. Modern breast radiotherapy techniques are largely ‘heart-sparing’, which is expected to avoid future problems for current patients.3,25 Monitoring heart health in cancer patients is well covered in a primary care guide produced by Macmillan26 and in the Guidelines in Practice article on Top tips: cardiotoxicity and cancer.
Dr Richard Simcock
Macmillan Consultant Medical Advisor and Oncologist
Dr Nicola Harker
Macmillan GP Adviser

