Dr Anne Connolly Outlines the Importance of Understanding Risk Factors and Early Opportunities to Intervene for Endometrial Hyperplasia and Cancer
| Read This Article to Learn More About: |
|---|
|
Endometrial cancer (also termed uterine or womb cancer) is the sixth most common malignant disorder in women worldwide.1 In the UK, the incidence of endometrial cancer has increased by approximately 60% since the early 1990s; between 2016 and 2018, approximately 9700 new cases of endometrial cancer were diagnosed each year.2 Over the past decade, age-standardised mortality rates for endometrial cancer have increased by 25% in the UK, where the condition is now the seventh most common cause of cancer death in women.2 Although it is predicted that more than 70% of women diagnosed with endometrial cancer in England will survive for 10 years or more,2 the condition was responsible for around 2500 deaths in 2018.2
There are several different types of uterine cancer, but this article focuses on the importance of risk recognition, management, and early referral for investigations of the most common type—endometrial adenocarcinoma.3
The Menstrual Cycle
The natural history of endometrial cancer is a progression from endometrial hyperplasia.4 An understanding of the menstrual cycle enables a logical approach to many gynaecological disorders, and can help to explain the risk factors that lead to the development of endometrial hyperplasia and, in turn, endometrial cancer (see Figure 1).5
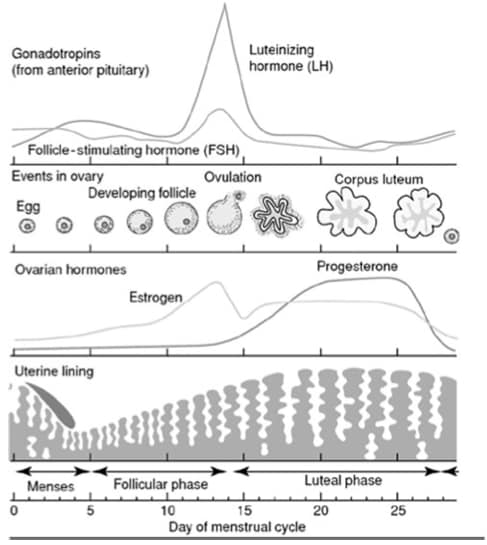
The menstrual cycle is controlled by the hypothalamic–pituitary–ovarian axis
GnRH is secreted by the hypothalamus, and stimulates the anterior pituitary to release the gonadotropins FSH and LH
In the follicular phase of the cycle, FSH stimulates the development of a few ovarian follicles, which consist of an oocyte surrounded by granulosa cells
In response to FSH, granulosa cells secrete oestradiol
One developing follicle matures to become dominant
Oestradiol secretion by the dominant follicle causes suppression of FSH production via a negative feedback mechanism
Oestradiol acts on the endometrium to stimulate proliferation
At a critical oestradiol level, there is a surge of LH, which triggers ovulation
The empty follicle transforms into the corpus luteum, which secretes progesterone in addition to oestradiol
The combination of oestradiol and progesterone develops the endometrium in preparation for implantation of a fertilised ovum
If conception does not occur, the corpus luteum degenerates and oestrogen and progesterone levels fall, causing endometrial cell death and menstruation.
GnRH=gonadotropin-releasing hormone; FSH=follicle-stimulating hormone; LH=luteinising hormoneDutton P, Rymer J. Physiology of the menstrual cycle and changes in the perimenopause. In: Managing the menopause—21st century solutions. Panay N, Briggs P, Kovacs G, editors. Cambridge: Cambridge University Press, 2015: 1–10. Reproduced with permission of Cambridge University Press through PLSclear.
Endometrial Hyperplasia
Fundamentally, the cause of endometrial hyperplasia is excess oestrogen when unopposed by the protective effects of progesterone.6 Endometrial hyperplasia is defined as ‘irregular proliferation of the endometrial glands with an increase in the gland to stroma ratio when compared with proliferative endometrium’, and can develop into endometrial cancer if left unmanaged.6,7
In 2014, the World Health Organization simplified the classification of endometrial hyperplasia based on the presence of cytological atypia as follows:6,7
- hyperplasia without atypia
- atypical hyperplasia.
In one study, hyperplasia without atypia progressed to endometrial carcinoma in fewer than 5% of women; in comparison, atypical hyperplasia progressed to endometrial carcinoma in one in eight women within 10 years, and in one in three women within 20 years.8
Endometrial Cancer
The majority of endometrial cancers are adenocarcinomas arising from the endometrial glands, and are diagnosed at an early disease stage.3
Figure 2 shows the proportion of patients diagnosed at different stages of endometrial cancer and their 5-year survival rates.9
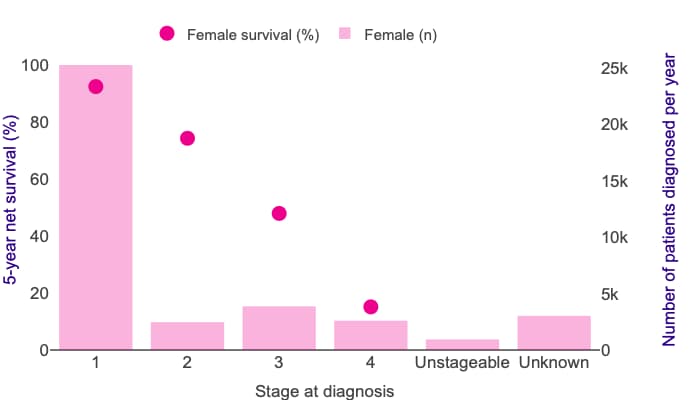
Endometrial cancer 5-year net survival by stage, with incidence by stage (all data: adults diagnosed 2013–2017, followed up to 2018).
Five-year net survival for endometrial cancer shows a large difference in survival between stages 1 and 4. In these females, 5-year net survival ranges from 92% at stage 1 to 15% at stage 4 for those diagnosed during 2013–2017 in England.
© Cancer Research UK. Uterine cancer survival statistics. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/survival-heading-Three
Reproduced with permission.
Risk Factors for Endometrial Hyperplasia and Cancer
Prompt recognition of risk factors for endometrial hyperplasia provides an opportunity for prevention, risk reduction, and early intervention (see Box 1).
| Box 1: Risk Factors for Endometrial Hyperplasia and Cancer |
|---|
Risk factors for endometrial hyperplasia and cancer are any condition in which there is excess oestrogen when unopposed by progesterone:
|
Age
The prevalence of endometrial cancer increases with age, and most women diagnosed with the condition have been through the menopause.10 Almost 75% of cases of endometrial cancer occur in women aged 40–74 years.10
Overweight and Obesity
An estimated one in three endometrial cancers in the UK is caused by overweight and obesity.10–12 In women with an increased body mass index, there is excessive peripheral conversion of androgens to oestrogen in adipose tissue,6,10 which is particularly relevant in postmenopausal women, in whom natural progesterone production is absent.
Anovulatory Cycles
Because natural progesterone is produced by the corpus luteum, anovulatory cycles that occur during the perimenopause,6 or in women with oligo- or amenorrhoea caused by polycystic ovary syndrome (PCOS),6,13 cause chronic unopposed oestrogenic stimulation of the endometrium. In women with PCOS, the risk of endometrial cancer is increased approximately 2.9-fold.13
Nulliparity
Nulliparity increases the risk of developing endometrial cancer by 42%.14 Although the exact mechanism by which nulliparity raises the risk of endometrial cancer is unknown, anovulatory cycles caused by certain types of infertility may contribute to this phenomenon.14
Diabetes
Women with diabetes have an increased risk of developing endometrial cancer.15 The mechanism underlying this elevated risk is not fully understood, but there is likely to be a link between insulin and endometrial cancer.
Exogenous Oestrogen Use
When treating menopausal women with an intact uterus with hormone replacement therapy (HRT), combination therapy (with both oestrogen and a progestogen) is required.16 Oestrogen-only HRT increases the risk of endometrial hyperplasia at all doses.17
The use of combined HRT is also essential for menopausal women after endometrial ablation, and should be considered for those with endometriosis post-hysterectomy because of the risk of residual endometrial deposits,18 which may be overstimulated by oestrogen-only HRT.
Tamoxifen Use
Tamoxifen is a selective oestrogen receptor modulator with an anti-oestrogenic effect in breast tissue, but a pro-oestrogenic effect on the endometrium.6 Use of tamoxifen increases the risk of endometrial cancer by around 2.5 times.19
Oestrogen-secreting Tumours
The prevalence of endometrial hyperplasia in women with oestrogen-secreting ovarian tumours—for example, granulosa cell tumours—is estimated to be 40%.6
Genetic Risk
Lynch syndrome, or hereditary nonpolyposis colorectal cancer, is estimated to cause about 3% of all endometrial cancers.20
Presentation
The most common symptom experienced by women with endometrial hyperplasia and cancer is postmenopausal bleeding (PMB),21 which is defined as unexplained vaginal bleeding occurring more than 12 months after menstruation has stopped because of the menopause.21
The probability that endometrial hyperplasia or cancer is the underlying cause of PMB is about 15%.22 The likelihood that cancer is the underlying cause of PMB increases with age.22
In premenopausal women, recognising when it is necessary to refer for investigations is harder because the symptoms experienced are common.6 These symptoms include:6
- heavy menstrual bleeding
- intermenstrual bleeding
- irregular bleeding.
As ever, a holistic approach to care alongside an awareness of the risk factors will aid in the recognition of when an early referral is needed.
Referral, Investigations, and Management
Referral
Physical examination is usually normal, but cervical abnormalities should be excluded by speculum examination to ensure prompt referral using the correct fast-track pathway.
NICE Guideline 12, Suspected cancer: recognition and referral, first published in 2015 and updated in 2021, lists the recommendations for referral when endometrial cancer is suspected in primary care (see Box 2).21
| Box 2: Recommendations for Referral for Suspected Endometrial c.Cancer21 |
|---|
© NICE 2021. Suspected cancer: recognition and referral. Available at: www.nice.org.uk/ng12 All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. See www.nice.org.uk/re-using-our-content/uk-open-content-licence for further details. |
Investigations
NICE states that hysteroscopy is the preferred investigation for endometrial assessment,23 allowing direct visualisation and sampling of any suspicious pathology. ‘Blind’ endometrial biopsy is not recommended;23 significant pathology may be missed using this technique.
The majority of women referred for endometrial assessment will be postmenopausal, and will be seen in the ‘one-stop’ PMB clinic, where scanning, consultation, and hysteroscopy are performed in one visit. A transvaginal ultrasound scan will provide details of ovarian pathology and an endometrial thickness measurement. Most specialist units are reassured by a ‘thin’ (3 or 4 mm) endometrial measurement, below which the probability of cancer is less than 1%.6 With endometrial measurements of more than 4 mm, one-stop hysteroscopy is recommended in the outpatient setting.6
Endometrial measurements in premenopausal women are less informative because endometrial thickness changes throughout the menstrual cycle.6 Therefore, the role of ultrasound in these women is to identify structural abnormalities,6 such as fibroids. Sometimes, an ultrasound scan will have been performed; the most concerning appearances warranting urgent referral for endometrial assessment are a thickened endometrium with a cystic appearance.
Any woman presenting with abnormal bleeding while using tamoxifen requires hysteroscopy, as visualisation and biopsy are required.23
Management
The joint Royal College of Obstetricians & Gynaecologists (RCOG) and British Society for Gynaecological Endoscopy (BSGE) green-top guideline on the management of endometrial hyperplasia was published in 2016.6 The guideline provides up-to-date, evidence-based recommendations on the risk factors, classification, diagnosis, and management of endometrial hyperplasia.
Endometrial Hyperplasia Without Atypia
Because the risk of progression of endometrial hyperplasia without atypia is extremely low, identification and management of reversible risk factors (such as obesity and HRT use) may be all that is required.6 However, women should be informed that progestogen treatment elicits a higher disease regression rate than observation alone.6 Active treatment is recommended for women with symptoms and a lack of regression after a period of observation.6
Progestogen treatment can be provided in the form of oral progestogens (medroxyprogesterone acetate 10–20 mg/day or norethisterone 10–15 mg/day), or via a levonorgestrel-releasing intrauterine system (LNG–IUS).6 An LNG–IUS is the preferred treatment because, compared with oral progestogens, it has a higher disease regression rate with a more favourable bleeding profile, and is associated with fewer adverse effects.6 For endometrial hyperplasia without atypia, treatment with oral progestogens or the LNG‑IUS should be for a minimum of 6 months.6
For women using tamoxifen who are diagnosed with endometrial hyperplasia without atypia, a treatment review should be undertaken by the gynaecologist and the woman’s oncology team.6
Hysterectomy is not recommended as a first-line treatment for endometrial hyperplasia without atypia.6 Endometrial ablation is also not recommended because of the risk of residual untreated endometrium.6
The 2021 Primary Care Women’s Health Forum and BSGE follow-up guidance outlines the shared-care arrangements and recommendations for follow up of women with endometrial hyperplasia without atypia (see Figure 3).24
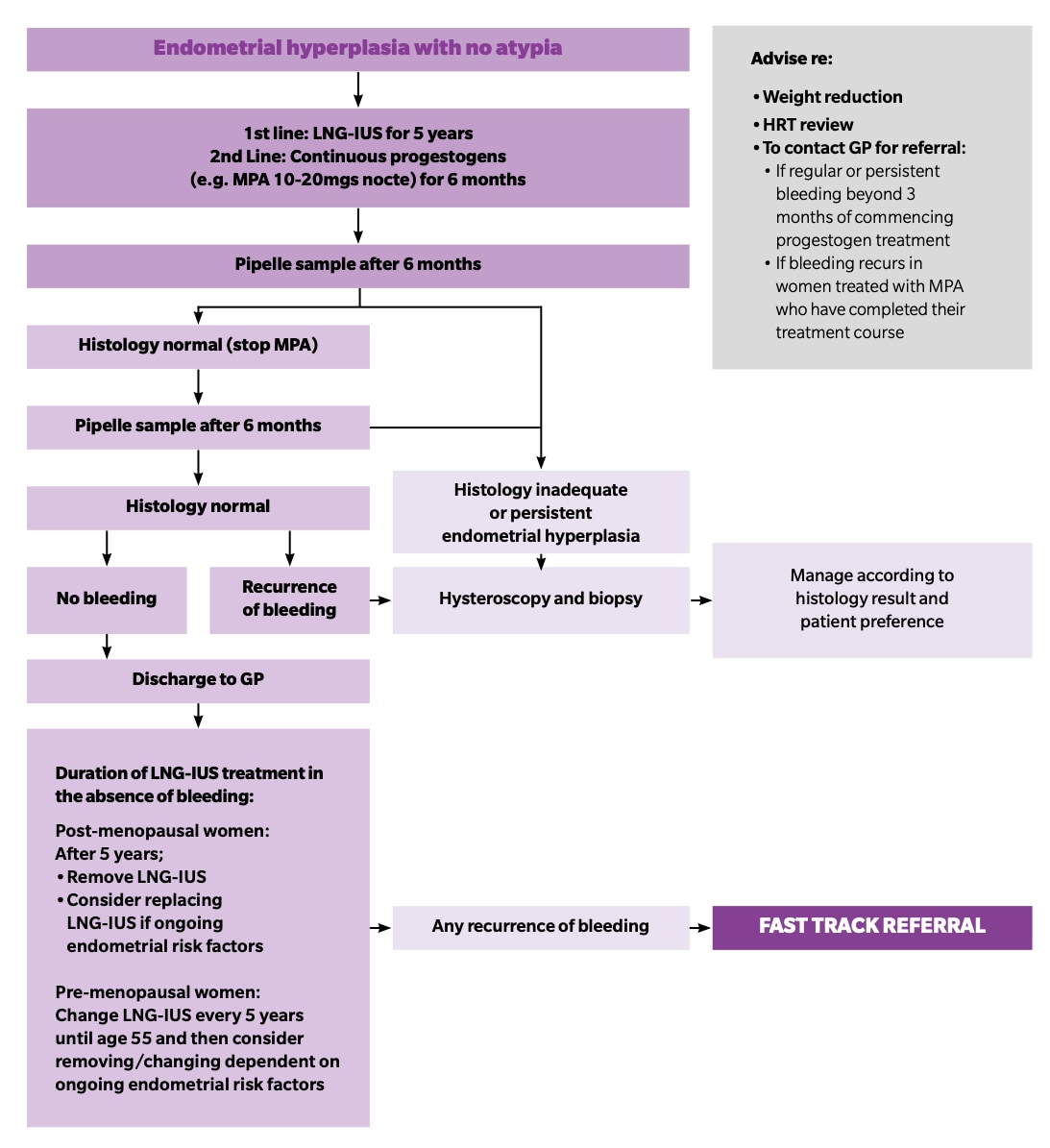
LNG-IUS=levonorgestrel-releasing intrauterine system; MPA=medroxyprogesterone acetate; nocte=at night; HRT=hormone replacement therapy
© Primary Care Women’s Health Forum. Management of endometrial hyperplasia with no atypia. Bedfordshire: PCWHF, 2021. Available at: pcwhf.co.uk/wp-content/uploads/2021/02/V3_Feb21_PCWHF_Endometrial-hyperplasia.pdf
Reproduced with permission.
Atypical Hyperplasia
Hysterectomy is the first-line management for atypical endometrial hyperplasia because of the high risk of coexisting malignancy or progression to cancer.6 In a study by the American Cancer Society, about 43% of patients with atypical hyperplasia had concurrent carcinoma.25
Women who are unsuitable for surgery or who wish to retain their fertility should be managed with progestogens—an LNG-IUS is the preferred option, with oral progestogens as a second-best alternative—and ongoing endometrial surveillance.6 For women wishing to conceive, improved pregnancy outcomes may be achieved using assisted conception following at least one endometrial sample showing disease regression.6
The Role of Primary Care in Prevention
An estimated one-third of cases of endometrial cancer are preventable.2 Identifying premenopausal women at higher risk of developing endometrial hyperplasia, and intervening earlier with weight reduction support and use of progestogen treatments, are important to reduce their risk of developing endometrial cancer.
Both oral and continuous (via an LNG-IUS) progestogen treatment reduce the risk of progression of endometrial hyperplasia.6 Progestogen treatment can be provided by any hormonal contraceptive (after risk assessment), but the RCOG/BSGE guideline recommends an LNG-IUS as the first-line option.6 To prevent endometrial hyperplasia from developing, women with oligo- or amenorrhoea due to PCOS should be managed with any progestogenic method of contraception or cyclical courses of a progestogen inducing a withdrawal bleed every 3 months (after excluding pregnancy).13
For postmenopausal women with an intact uterus, correct HRT prescribing, with a sufficient duration and dose of a progestogen in the regimen, is essential to reduce the risk of endometrial hyperplasia and cancer.26
Women receiving progestogen treatment following a diagnosis of endometrial hyperplasia are discharged from specialist units after two consecutive negative biopsies. It is essential that ongoing progestogen treatments are prescribed for a minimum of 6 months, and a diary entry made to re-refer for 6-monthly follow-up biopsies if appropriate.6
Summary
Endometrial cancer is increasing in incidence, and in one-third of cases it is preventable.2 The precursor to this cancer is endometrial hyperplasia, which may be responsive to risk factor reversal and conservative management with progestogens.6
The majority of cases of endometrial cancer occur in postmenopausal women, who commonly present with PMB.10,27 Pathways for referral are clearly outlined in the NICE suspected cancer guidance.21
Endometrial hyperplasia and cancer are harder to recognise in premenopausal women, as the presenting symptoms may be similar to other abnormal bleeding patterns. An appreciation of endometrial risk factors—and a holistic approach to determining who can be managed, and who should be referred for further investigation—are essential to early detection of endometrial hyperplasia and reducing endometrial cancer rates.
| Key Points |
|---|
PMB=postmenopausal bleeding |
Dr Anne Connolly
GPwSI in Gynaecology, Bevan Healthcare, Bradford; Chair of the Primary Care Women’s Health Forum; and member of the Guidelines in Practice Editorial Advisory Board

