Dr Glenis Scadding Describes an Algorithm that Summarises how to Actively Manage Allergic Rhinitis to Improve Symptoms in People with Asthma
| Read This Article to Learn More About: |
|---|
Find implementation actions for STPs and ICSs at the end of this article |
Most people with asthma also have some form of upper airway inflammatory disease, either rhinitis or rhinosinusitis.1 Those without rhinitis or rhinosinusitis probably do not have type 2 eosinophilic asthma, which may not respond to corticosteroid treatment, so asking about the nose and sinuses can help to categorise asthma.
However, there are better reasons for identifying nasal problems: not only do rhinitis symptoms reduce quality of sleep and, therefore, quality of life, and work/school performance, but rhinitis also has significant multimorbidities involving the sinuses, throat, ears, and chest.2 Rhinitis, both allergic and non-allergic, can evolve into asthma, worsen control of existing asthma, and increase GP visits and asthma costs.3–5 In children and adults with asthma the likelihood of needing hospital treatment is more than doubled by the most significant risk factor: the presence of allergic rhinitis (AR).6,7
Patients rarely understand the unity of the respiratory tract: it has one ‘wallpaper’ of respiratory epithelium from just inside the nose down to the alveoli and an insult to any one part reverberates around the whole. Allergen deposited in the nose experimentally causes bronchial inflammation and bronchial hyperreactivity; the converse is also true.8 Many patients with asthma do not think to mention that their seasonal AR makes them less able to exercise without coughing and wheezing. Persistent AR can be missed altogether as a diagnosis as the major symptoms are nasal obstruction, often with postnasal catarrh, and reduced sense of smell.9 Even when brought to the attention of their GP, these symptoms are often mistakenly ascribed to a nasal structural fault, such as a deviated septum, and referral, if it happens, is to an ear, nose, and throat (ENT) surgeon.
However, treating AR well benefits concomitant asthma.5 So how can the busy GP or asthma nurse go about effective treatment? My colleagues Dermot Ryan, Steve Holmes, Andrew Williams, and I have given thought to facilitating the process by devising an algorithm (Figure 1).10
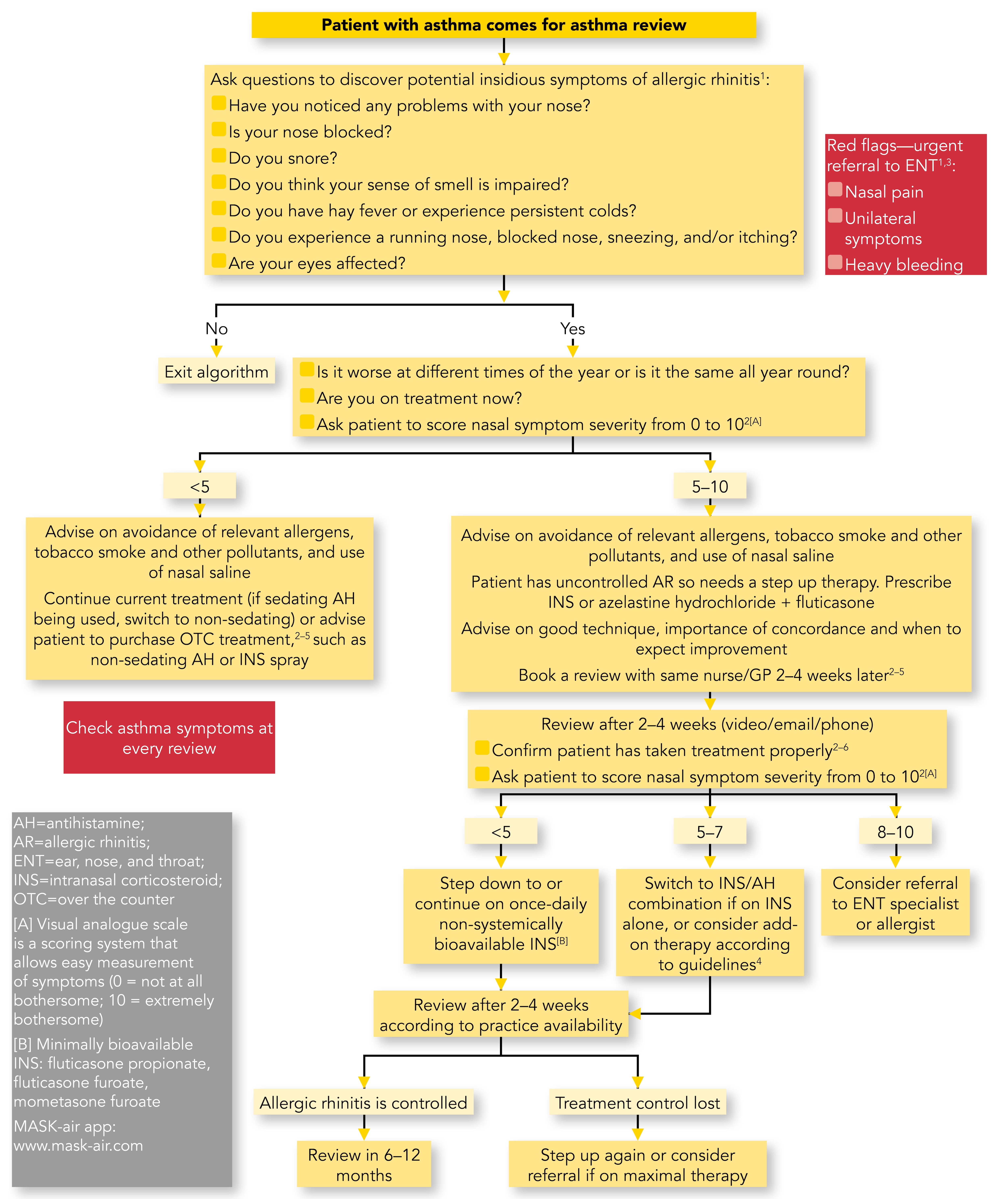
NB: See below for references associated with this algorithm
Scadding G, Holmes S, Ryan D, Williams A. Identifying and managing allergic rhinitis in the asthma population. In: Hatton S, editor. Guidelines—summarising clinical guidelines for primary care. 76th edition. Chesham: MGP Ltd, 2020. pp.222–225.
References
1. Scadding G et al. Prim Care Respir J 2012; 21 (2): 222–228.
2. Bousquet J et al. J Allergy Clin Immunol 2016; 138 (2): 367–374.
3. NICE. CKS: Allergic rhinitis. NICE, 2018. Available at: www.cks.nice.org.uk/allergic-rhinitis
4. Scadding GK et al. Clin Exp Allergy 2017; 47 (7): 856–889.
5. Brozek J et al. J Allergy Clin Immunol 2016; 140 (4): 950–958.6. Del Cuvillo A. Rhinology 2017; 55 (1): 34–38.
Diagnosis
The algorithm recommends that at asthma review a few more questions are asked. First, are there any problems with your nose? Second, more detailed questions about blockage, snoring, and smell impairment to identify patients with persistent rhinitis or rhinosinusitis. Finally, there are three questions relating to seasonal rhinitis: hay fever, nasal running, itching, sneezing, and eye involvement. Persistent colds are included here as sometimes AR is misdiagnosed by the patient.
Nasal pain, unilateral symptoms, or heavy nasal bleeding are red flags that should prompt urgent referral to ENT. Snoring that is severe enough to cause sleep apnoea also warrants referral to a sleep clinic with advice in the meantime on weight loss (for those who are overweight or obese), avoiding alcohol, sleeping pills, and tobacco smoke, sleeping on their side, and not eating after 19.00.
If the answers to all the questions are negative, the patient does not have rhinitis or rhinosinusitis.
If the answers are all positive, further questions involve timing, current treatment, and nasal symptom severity scoring using a simple visual analogue scale (VAS) from 0 to 10, with 0 indicating no current symptoms and 10 the worst possible.11
All patients with rhinitis should be advised to avoid relevant allergens, tobacco smoke, and other pollutants, and of the benefits of saline nasal douching.12,13
Medication
For patients scoring less than 5 on a VAS, a level indicating control,11 current medication should be continued unless it is a sedating antihistamine, in which case a switch to a non-sedating antihistamine or an intranasal corticosteroid (INS) spray is necessary. Sedating antihistamines worsen the psychomotor retardation of rhinitis and decrease work and school performance and driving ability, as well as having more potential for cardiac arrhythmias.14
Patients scoring more than 5 on a VAS have uncontrolled rhinitis and need initiation of therapy with an INS or a step-up in existing INS therapy to a combination of INS plus intranasal antihistamine (see Figure 2).13 Adding an oral antihistamine to an INS does not confer additional benefit.13
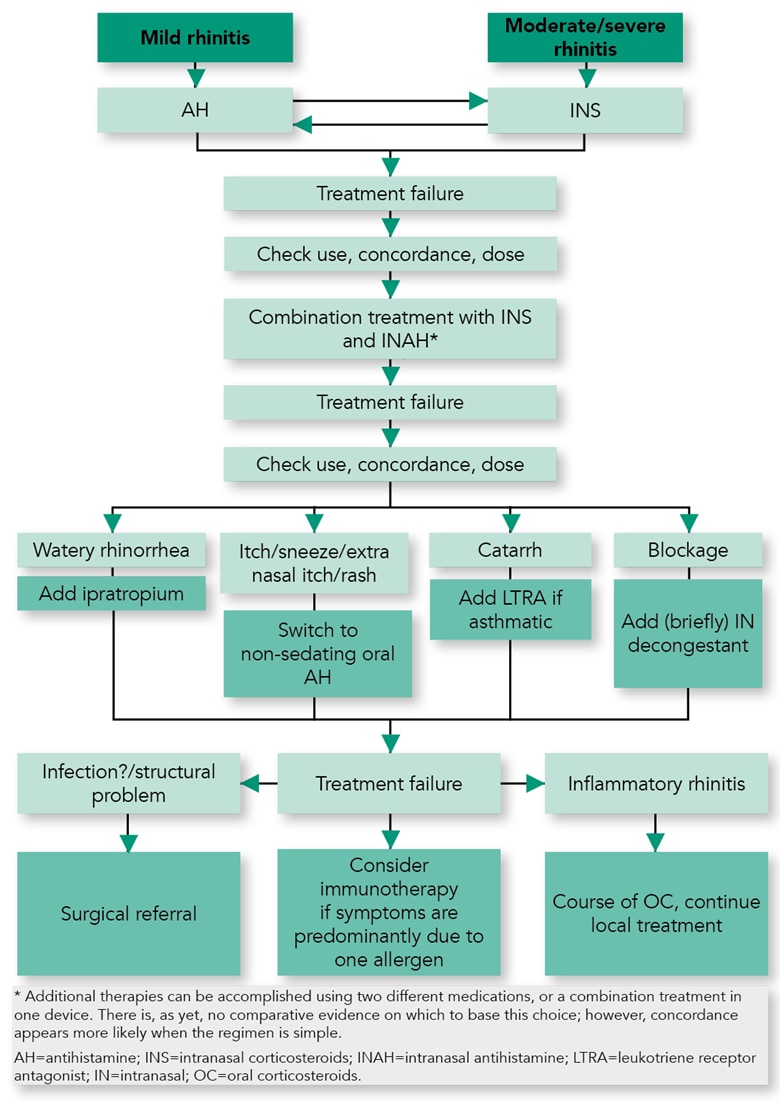
Adapted from: Scadding GK, Kariyawasam H, Scadding G et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First Edition 2007). Clin Exp Allergy 2017; 47 (7): 856–889. Reproduced with permission
It is important to show patients the correct use of a nasal spray (see Figure 3), to explain that regular prophylactic use is needed, and that benefit from INS is not immediate and can take 2 weeks. Patients should be warned about possible side-effects such as epistaxis with INS, or a bitter taste with azelastine. Worries about corticosteroid load are unnecessary, provided that one of the minimally bioavailable INS (fluticasone propionate, fluticasone furoate, or mometasone furoate) is used.13
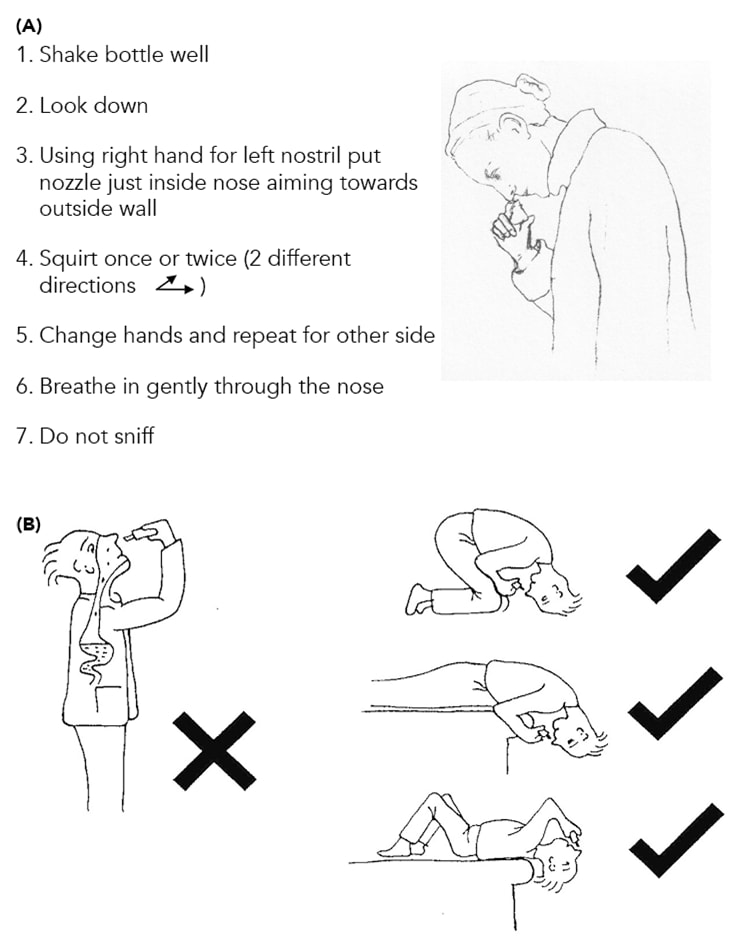
Adapted from: Scadding GK, Kariyawasam H, Scadding G et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First Edition 2007). Clin Exp Allergy 2017; 47 (7): 856–889. Reproduced with permission
Review
Review after 2–4 weeks by telephone or video link is advisable to confirm if the treatment has been taken correctly and assess its effectiveness by a repeat VAS score. At each review, asthma symptoms and home peak flows or spirometry should also be checked. If there is improvement with successful AR treatment, asthma medications could be stepped down.
Patients whose symptoms are now controlled (VAS less than 5) could be stepped down to, or continued on, therapy with an INS if symptoms are persistent or if their pollen season continues.
Scores between 5 and 7 inclusive suggest the need for a switch to an INS/H1-antihistamine combination in those on INS alone or additional guideline-directed treatment, such as montelukast.13 Further review after 2–4 weeks is recommended, leading either to 6 months of therapy if symptoms are controlled, or stepping up therapy or referral if uncontrolled.
Scores of 8–10 suggest the need for referral to an ENT surgeon if structural problems exist (an otoscope can be used to examine the nose from below, ensuring COVID-19 precautions are taken). New polyps should be referred to an ENT surgeon. Referral to an allergist is sensible if uncontrolled inflammatory disease is present, including recurrent known eosinophilic polyps.15 Allergen-specific immunotherapy (AIT) is the only disease-modifying treatment and should probably be more widely used. AIT given for AR, either subcutaneous or sublingual, may also benefit lower airway function and reduce the development of asthma/asthma symptoms in children and adults with AR.16,17 The European Academy of Allergy and Clinical Immunology guideline on prevention of allergy states:
‘Our key recommendation is that a 3-year course of subcutaneous or sublingual AIT can be recommended for children and adolescents with moderate-to-severe allergic rhinitis (AR) triggered by grass/birch pollen allergy to prevent asthma for up to 2 years post-AIT in addition to its sustained effect on AR symptoms and medication.’ 17
Monoclonal antibodies, such as anti-immunoglobulin-E, anti-interleukin-5, and anti-interleukin-4/13 receptor therapy, also show promise for treating patients with co-morbid asthma and chronic rhinosinusitis with nasal polyps in those with symptoms at the severe end of the spectrum.18
Mobile phone apps such as MASK-air (www.mask-air.com) now exist for monitoring and can act as useful reminders to take medication and to record symptoms of both asthma and rhinitis.19
Corticosteroid absorption is much greater from the lungs than from the nose, because of their large absorptive area, so using part of the corticosteroid dose in the nose to aid asthma control is sensible. Teaching patients to exhale their inhaled corticosteroid via the nose is sensible, but insufficient.
Summary
Allergic rhinitis is often ignored in people with asthma. However, there are benefits for both the upper and lower airway and to quality of life from effective allergic rhinitis management.
The approach presented in this article should improve recognition and provide evidence-based treatment of AR in people with asthma, and mobile monitoring is suggested where possible to aid concordance. Our aim is to maximise patient benefit and asthma control by including the whole airway in therapy, without adding to the burden of systemic corticosteroid exposure.
Dr Glenis Scadding
Honorary Consultant Physician at the Royal National ENT Hospital, UCL Hospitals, London
Member of the guideline development group for the Guidelines working party algorithm on identifying and managing allergic rhinitis in the asthma population
| Implementation Actions for STPs and ICSs |
|---|
Written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved with implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system; ENT=ear, nose, and throat |

