Dr Clare Hambling and Dr Patrick Holmes highlight the key recommendations from the joint ADA/EASD consensus report on the management of hyperglycaemia in type 2 diabetes
| Read This Article to Learn More About: |
|---|
Find key points and implementation actions for STPs, ICSs, and clinical pharmacists in general practice at the end of this article
  |
The worldwide prevalence of type 2 diabetes continues to rise, with diet, sedentary lifestyle, and overweight and obesity considered the main contributing risk factors.1 In the UK, the number of people diagnosed with diabetes has more than doubled in the past 20 years and an estimated 4.7 million people now live with diabetes, 90% of whom have type 2 diabetes.2
Living with type 2 diabetes has important implications for affected individuals and for healthcare resources. In 2012, expenditure for type 1 and type 2 diabetes was estimated to account for 10% of the NHS budget and was projected to rise to 17% by 2035–36.3 Approximately 80% of this cost relates to the management of diabetes-related complications.3 Despite improvements in care, and evidence that early intensive management reduces the risk of complications and improves life expectancy,4 people living with diabetes remain at risk of long-term complications, morbidity, and premature mortality.5
In the UK, atherosclerotic cardiovascular disease (ASCVD), including acute coronary syndromes, heart failure (HF), stroke, transient ischaemic attack, and peripheral arterial disease, which contributes to diabetic foot disease and the risk of lower limb amputation, remain a particular concern,6–8 and diabetes is one of the most common causes of chronic kidney disease (CKD) and end-stage renal disease.9 Severe hypoglycaemia, a complication of glucose-lowering treatment, results in emergency ambulance call outs10 and unplanned hospital admissions,11 with older people most commonly affected10,11 and at increased risk of consequential injury, harm, and associated increased likelihood of mortality.12
Guidelines for Management of Type 2 Diabetes
Previous guidelines for the management of type 2 diabetes often focused on metabolic parameters but, increasingly, there is an understanding of the need for a more holistic approach. People living with diabetes represent a diverse population; type 2 diabetes affects adults of all ages and from all cultural backgrounds. Individuals have varying clinical characteristics and co-morbidities and face very different life challenges. Additionally, interventional trials in type 2 diabetes now commonly focus on outcomes beyond glycaemic parameters, in particular the benefits and risks for co-morbid conditions such as obesity, ASCVD, CKD, and hypoglycaemia, and this offers the potential for a more individualised approach to glycaemic management. Consequentially, goals and therapeutic choices can be person-centred and aimed at treating symptomatic hyperglycaemia, reducing the risk of long-term complications, and maintaining general health, wellbeing, and quality of life in a holistic context.
ADA/EASD Consensus Report
The 2018 consensus report and recommendations for management of hyperglycaemia in type 2 diabetes from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD),13 is an update on the previous 2015 position statement. It is based on a review of the current best available evidence, with particular reference to some of the common co-morbidities associated with type 2 diabetes. The report emphasises a holistic, person-centred approach, lifestyle management, and the selection of glucose-lowering therapies based on individual characteristics and co-morbidity.13
Rationale
In preparing for the 2018 position statement, the writing group reviewed recently published evidence, covering both non-pharmacological and pharmacological interventions in type 2 diabetes, developing consensus recommendations and guiding principles for providing care. The consensus recommendations are summarised in Box 1.13
| Box 1: Consensus Recommendations from the 2018 ADA/EASD Position Statement13 |
|---|
ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes; DSMES=diabetes self-management education and support; ASCVD=atherosclerotic cardiovascular disease; BMI=body mass index; CKD=chronic kidney disease; CVD=cardiovascular disease; GLP-1 receptor agonist=glucagon-like polypeptide-1 receptor agonist; HF=heart failure; SGLT-2=sodium-glucose co-transporter-2 |
Fundamentals of Diabetes Care
The importance of dietary advice, weight management, physical activity, smoking cessation, and psychological support are acknowledged as fundamental elements in providing diabetes care, as too is the need for person-centred care and shared decision making. This position statement from ADA/EASD moves away from generalisable treatment targets, recommending individualised glycaemic goals, based on patient preferences, clinical characteristics, co-morbidity, and the risk of adverse medication effects (e.g. hypoglycaemia or weight gain). In promoting shared decision making, ADA/EASD advises clinicians to be culturally sensitive and to consider factors such as health beliefs, literacy, cognitive impairment, and the impact of recommended interventions on self-care.13
Diabetes Self-management Education and Support
Diabetes self-management education and support (DSMES) is highlighted in the consensus report as key to enabling informed decision making and empowering individuals to assume responsibility for day-to-day management of their diabetes. Providing structured education is considered cost-effective and leads to improvements in patient knowledge, clinical and psychological outcomes, healthy eating, physical activity, medication adherence, glycaemic control, as well as reduced hospital admissions and all-cause mortality. The evidence base remains stronger for face-to-face education, which is preferred, although digital solutions are considered useful to support learning. The ADA/EASD consensus report recommends that the DSMES:13
- is evidence-based
- is individualised to reflect the needs of the person, including language and culture
- has a structured, theory-driven, written curriculum with supporting materials
- is delivered by trained and competent individuals (educators) who are quality assured
- is delivered in a group or individual settings
- aligns with the local population needs
- supports the person and their family in developing attitudes, beliefs, knowledge, and skills to self-manage diabetes
- includes core content, such as:
- diabetes pathophysiology and treatment options
- medication usage
- monitoring, preventing, detecting, and treating acute and chronic complications
- healthy coping with psychological issues and concerns
- problem solving and dealing with special situations (e.g. travel, fasting)
- is available to patients at critical times (i.e. at diagnosis, annually, when complications arise, and when transitions in care occur)
- includes monitoring of patient progress, including health status, quality of life
- is quality audited regularly.
The Decision Cycle
Central to the ADA/EASD position statement are attractive and easy to follow algorithms, including a decision cycle for patient-centred glycaemic management (see Figure 1 of reference 13).13 The overarching goals of care are to prevent complications and optimise quality of life. The person with diabetes is placed firmly at the centre of a cycle of care that promotes holistic assessment and shared decision making to arrive at an agreed management plan. The plan is shaped around the individual, considering their personal preferences, clinical characteristics, and co-morbidities, the benefits and risks of glucose-lowering medications, the need for monitoring and support, as well as planned review.13
Lifestyle Management
Lifestyle management is recommended as first-line therapy but should also be part of the ongoing discussion at each visit to the GP. Diet and physical activity are effective and safe for improving glucose control in type 2 diabetes, with a combination of the two being better than either one alone. Various physical activities are beneficial, with aerobic exercise and resistance training, as well as combination training, improving glycaemic control.13
Diet and Weight
The consensus report summarises evidence from dietary interventions, concluding that many different diets have evidence for beneficial weight loss and glycaemic improvement and, therefore, no specific diet is recommended.8,13 Dietary advice is generalisable, should acknowledge individual preferences, and be aimed at supporting healthy dietary habits that are feasible and sustainable, with emphasis on choosing foods with health benefits and minimising consumption of foods with evidence of harm.13
All overweight and obese people with diabetes should be advised of the health benefits of weight loss and encouraged to engage in a programme of intensive lifestyle management, with food substitution and bariatric surgery considered options for some. In people who have had diabetes for less than 6 years, non-surgical weight loss of 10–15 kg has recently been associated with diabetes remission.14
ADA/EASD Recommendations for Pharmacological Therapy for Type 2 Diabetes
In line with current NICE guidance for the management of type 2 diabetes in adults,15 the 2018 ADA/EASD consensus statement recommends metformin as the first-line glucose lowering agent.13 Thereafter, the selection of glucose-lowering therapies depends on the patient’s individual characteristics and co-morbidity.13
Metformin
Unless contraindicated or not tolerated, metformin remains the first-line treatment of choice, in conjunction with comprehensive lifestyle advice. As highlighted in the UK Prospective Diabetes Study (UKPDS) of 2008,16 whether metformin confers cardiovascular benefit is uncertain,17 but it is effective in lowering HbA1c, with a good safety profile, low incidence of hypoglycaemia, beneficial effects on weight, and low cost.13 Instructions for use, side-effects, and cautions for metformin use, as detailed in the ADA/EASD position statement, are summarised in Box 2.13
| Box 2: Metformin Dose, Side-effects, and Cautions for Use as Detailed by ADA/EASD13 |
|---|
Dose
Side-effects
Cautions for use
ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes; eGFR=estimated glomerular filtration rate |
Second-line Therapy After Metformin
An exciting new element introduced in the ADA/EASD consensus statement is the selection of second-line and subsequent glucose-lowering therapies based on co-morbidity, individual clinical characteristics, risk of harm from potential medication side-effects, and socioeconomic factors. A few key questions guide therapeutic recommendations:13
- does the individual have ASCVD (such as ischaemic heart disease, cerebrovascular or peripheral vascular disease)?
- does the individual have HF or CKD?
- is there a compelling reason to avoid weight gain or promote weight loss?
- is there a compelling reason to avoid hypoglycaemia?
- is medication cost the major issue?
An affirmative answer (‘yes’) guides the choice of glucose-lowering therapies by directing the clinician through a series of algorithms.13 At every point, lifestyle advice and the need to avoid clinical inertia (i.e. failure to intensify therapy when treatment targets are not met) is highlighted, along with the need for regular reassessment and adjustment of therapies.13
In determining their recommendations, the writing group considered new evidence from a large number of cardiovascular outcomes trials (CVOT), which is summarised in the consensus report. An algorithm summarising the overall approach to glucose-lowering medications in type 2 diabetes is presented in Figure 1. This approach highlights the potential for added benefits from specific sodium glucose co-transporter 2 (SGLT-2) inhibitors or glucagon-like peptide 1 receptor agonists (GLP-1 receptor agonist), with improved ASCVD outcomes and beneficial effects on HF and CKD, as well as the impact of therapeutic choice on weight, risk of hypoglycaemia, and cost.13 The text below should be read in conjunction with the fuller details given in Figure 1.
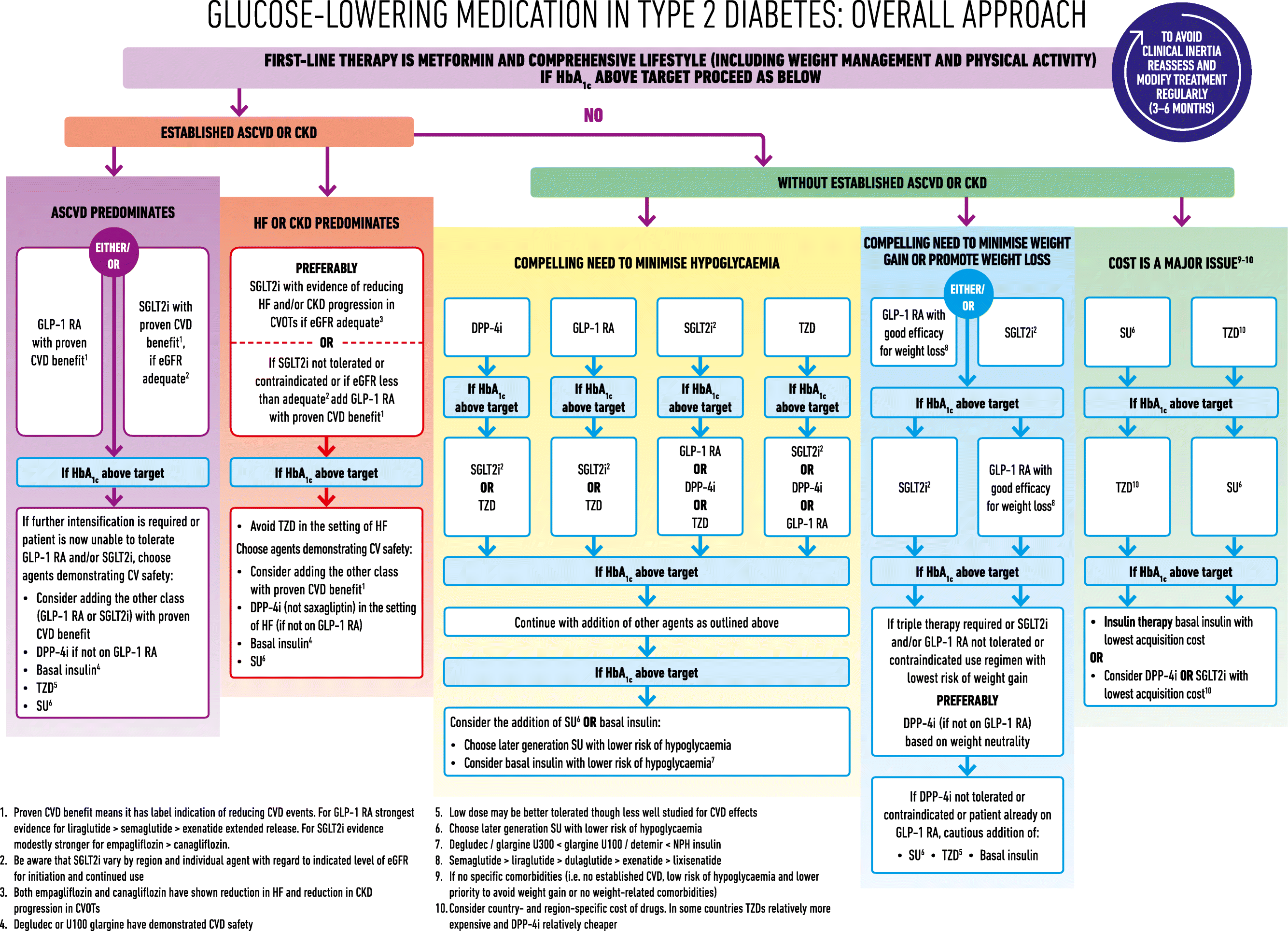
Source: Davies M, D’Alessio D, Fradkin J et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61: 2461–2498. Reproduced with permission
Established ASCVD Predominates
- Either a GLP-1 receptor agonist or SGLT-2 inhibitor (if eGFR is adequate) with proven CVD benefit is advised as the second-line agent13
- If further intensification is required or the patient can no longer tolerate a GLP-1 receptor agonist and/or SGLT-2 inhibitor, subsequent add-on therapies include:13
- GLP-1 receptor agonist or SGLT-2 inhibitor (the other class after the above) with proven CVD benefit
- dipeptidyl peptidase 4 inhibitor (DPP-4 inhibitor) if the patient is not on GLP-1 receptor agonist
- basal insulin
- thiazolidinedione (TZD; pioglitazone)
- sulfonylurea (SU).
Established HF or CKD Predominates
- An SGLT-2 inhibitor which has been shown by CVOT to reduce HF and/or CKD progression is the preferred second-line agent (if eGFR is adequate), with a GLP-1 receptor agonist with proven CVD benefit considered as an alternative option if an SGLT-2 inhibitor is not tolerated or is contraindicated. TZDs (pioglitazone) should be avoided in people with HF13
- If further intensification is required, subsequent add-on therapies include:13
- the other class with proven CVD benefit (i.e. GLP-1 receptor agonist or SGLT-2 inhibitor)
- DPP-4 inhibitor (with the exception of saxagliptin, which should be avoided in HF), if the patient is not on GLP-1 receptor agonist
- basal insulin
- SU.
NB The British National Formulary currently advises caution with the use of SGLT-2 inhibitors in co-morbid HF or CKD. Use of an SGLT-2 inhibitor may risk volume depletion, particularly in conjunction with loop diuretics; current licensed indications advise against initiation of an SGLT-2 inhibitor at eGFR <60 ml/min/1.73 m2 and advise discontinuation at eGFR <45 ml/min/1.73 m2.18–21 This means that for most people with renal impairment, a GLP-1 receptor agonist is currently the recommended second-line glucose-lowering therapy after metformin.
No Established ASCVD or CKD: Compelling Need to Minimise Hypoglycaemia
- Any of the following are considered options:13
- DPP-4 inhibitor—followed by SGLT-2 inhibitor or TZD if HbA1c remains above target
- GLP-1 receptor agonist—followed by SGLT-2 inhibitor or TZD if HbA1c remains above target
- SGLT-2 inhibitor (if eGFR is adequate)—followed by GLP-1 receptor agonist or DPP-4 inhibitor or TZD if HbA1c remains above target
- TZD—followed by SGLT-2 inhibitor or DPP-4 inhibitor or GLP-1 receptor agonist if HbA1c remains above target.
As in previous ADA/EASD position statements, the rationale is to avoid SU or insulin therapies, where possible. However, further down the algorithm (see Figure 1), where SU or insulin is required, specific reference is given to selection of second-generation SU (such as gliclazide or glimepiride), or basal insulin with lower risk of hypoglycaemia.13
No Established ASCVD or CKD: Compelling Need to Minimise Weight Gain or Promote Weight Loss
- SGLT-2 inhibitor if eGFR is adequate—followed by GLP-1 receptor agonist with good efficacy for weight loss13 or
- GLP-1 receptor agonist with good efficacy for weight loss—followed by SGLT-2 inhibitor if eGFR is adequate13
- If triple therapy is required, or SGLT-2 inhibitor or GLP-1 receptor agonist contraindicated or not tolerated, use regimen with lowest risk of weight gain, preferably DPP-4 inhibitor (if patient not on GLP-1 receptor agonist)13
- If DPP-4 inhibitor not tolerated or contraindicated, or patient already on GLP-1 receptor agonist, then SU, TZD, or basal insulin may be added cautiously as subsequent therapeutic options where necessary.13
This recommendation varies slightly from current NICE guidance on Type 2 diabetes in adults: management, where GLP-1 receptor agonists are positioned as options at second or third intensification only.15 The ADA/EASD position statement also guides choice of GLP-1 receptor agonist in terms of efficacy at promoting weight-loss, with semaglutide having the greatest effect and lixisenatide the least (i.e. semaglutide >liraglutide >dulaglutide >exenatide >lixisenatide).
Triple combination therapy with metformin, SGLT-2 inhibitor, and a GLP-1 receptor agonist is not currently recommended by NICE.15
No Established ASCVD or CKD: Cost of Medication is a Major Issue
Cost remains an important consideration for many individuals and health economies around the world. Because of this, the ADA/EASD writing group acknowledges the importance of financial considerations within the treatment algorithms.13
- SUs and TZDs are currently relatively inexpensive and, therefore, the recommended options where cost is a concern are:13
- SU followed by TZD
- TZD followed by SU
- If HbA1c remains high, use:13
- basal insulin with lowest acquisition cost or
- consider DPP-4 inhibitoror SGLT-2 inhibitor, with lowest acquisition cost
- Prices may vary over time and treatment options for reasons of cost may need regular review.
Glucose-lowering Therapies
- After agreeing initial glycaemic goal, early use of combination glucose-lowering therapies is generally advised where HbA1c is >17 mmol/mol (1.5%) above target13
- Cycles of early and repeated review are advised, with potentiation of glucose-lowering therapies until glycaemic goals are achieved13
- Where adding glucose-lowering therapies, it may be necessary to reduce the dose or discontinue other glucose-lowering therapies to avoid hypoglycaemia, particularly when adding a new agent to a regimen containing insulin, SU, or glinide therapy, especially in people at or near glycaemic goals13
- Throughout the algorithm (see Figure 1), advice is given to avoid the use of GLP-1 receptor agonist and DPP-4 inhibitor therapies in combination.13
Injectable Therapies
Type 2 diabetes is typically a progressive condition, which over time leads to beta-cell failure and the need to intensify to injectable therapies. As a general principle, the 2018 position statement from the ADA and EASD, recommends a GLP-1 receptor agonist as the preferred initial injectable therapy for most people. The rationale behind this guidance is to limit excessive weight gain and minimise the risks of hypoglycaemia.13 Whether this will be considered affordable for all people requiring injectable therapy or for all health economies is questionable.
For people where type 1 diabetes is a possibility, for example, where catabolic symptoms predominate (weight loss, polyuria, polydipsia), suggesting insulin deficiency, or where the HbA1c is >97 mmol/mol, then insulin should be considered as the first-line injectable therapy.13
If glycaemic goals are not met despite the addition and titration (if applicable) of the GLP-1 receptor agonist, then intensification with a basal insulin is recommended alongside the GLP-1 receptor agonist. Following the addition of basal insulin therapy, a step-wise introduction of prandial insulin, starting with the largest meal of the day, is recommended. Premixed insulins are not routinely recommended.13
Summary
The 2018 position statement from the ADA and EASD offers an evidence-based yet pragmatic approach to glycaemic management in people with type 2 diabetes. Throughout, the emphasis on holistic, person-centred care, with consideration of individual characteristics, co-morbidities, and socioeconomic factors, very much aligns with the challenges of providing diabetes care in the primary care setting within the UK. The position statement supports clinicians in offering an individualised approach, which is likely also to appeal to people living with type 2 diabetes.
Dr Clare Hambling
GP Principal, Norfolk
GP Clinical Lead for Diabetes, Norfolk and Waveney
Chair, Primary Care Diabetes Society
Professional member and Clinical Champion, Diabetes UK
Dr Patrick Holmes
GP Principal, Darlington
GPwSI County Durham & Darlington Foundation Trust
NIHR CRN North East Primary Care Diabetes Lead
Darlington CCG Diabetes Clinical Lead
Committee member, Primary Care Diabetes Society
| Key Points |
|---|
DSMES=diabetes self-management education and support programmes; ASCVD=atherosclerotic cardiovascular disease; HF=heart failure; CKD=chronic kidney disease; SGLT-2=sodium-glucose co-transporter-2; GLP-1 receptor agonist=glucagon-like polypeptide-1 receptor agonist; CVD=cardiovascular disease |
| Implementation Actions for STPs and ICSs |
|---|
Written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved with implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system; ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes; GLP-1=glucagon-like peptide-1; QOF=quality and outcomes framework; DVLA=Driver and Licensing Agency |
| Implementation Actions for Clinical Pharmacists in General Practice |
|---|
Written by Gupinder Syan, Training and Clinical Outcomes Manager, Soar Beyond Ltd The following implementation actions are designed to support clinical pharmacists in general practice with implementing the guidance at a practice level.
HbA1c=glycated haemoglobin; BP=blood pressure; ADA=American Diabetes Association; EASD=European Association for the study of Diabetes; HCPs=healthcare professionals; HF=heart failure; CKD=chronic kidney disease |

