Dr David Strain Outlines Six Key Learning Points From an Expert Consensus Statement on Managing Diabetes and Frailty, and Identifies Where Treatment Should Differ From a Standard Approach
| Read This Article to Learn More About: |
|---|
Find key points and implementation actions for STPs and ICSs at the end of this article |
Rates of population ageing are unprecedented and, combined with the progressive urbanisation of lifestyles, have led to a dramatic increase in diabetes in older adults. Both ageing and diabetes are recognised as important risk factors for the development of functional decline and disability (termed frailty), which is often compounded by impaired quality of life.1 In addition, diabetes is associated with a high economic, social, and health burden. In younger adults, macrovascular and microvascular complications of diabetes account for most morbidity and mortality, but in older adults these complications account for less than half of diabetes-related disability. Cognitive decline, frailty, and muscle loss (sarcopenia) are emerging as important new complications of diabetes, with a big impact on quality and quantity of life in these older adults.2–4
The Need for a Guidance Document
In 2019–2020, 53.1% of type 2 diabetes registrations for England and Wales combined occurred in those aged 65 and older;5 despite this, little is known about how best to treat this population. National and international guidelines for management are often extrapolated from trials performed in younger and/or healthier individuals.6 This approach may not be appropriate given the fundamental physiological differences between older adults (including those who are fit) and their frailer counterparts, such as less body mass, different distribution of fat tissue, and reduced production of and sensitivity to counter-regulatory hormones that protect against hypoglycaemia.
In 2018, guidance was published by a national UK collaborative stakeholder group to promote the assessment of frailty and the individualisation of treatment targets for older adults with type 2 diabetes.7 The guidance was rapidly adopted by NICE, and two new indicators—NM158 and NM159—were added to the indicator menu for general practice.8,9 In addition, a number of new indicators for diabetes that took account of frailty were included in the 2019–2020 General Medical Services contract Quality and Outcomes Framework.10 Although these actions provided a structure for what needed to be done, they did not indicate how it could be achieved. To address this, in 2021 a stakeholder group with representation from the Primary Care Diabetes Society and the Foundation for Diabetes Research in Older People, and with input from community nursing and hospital diabetologists and geriatricians, produced a consensus statement to facilitate the implementation of its guidance.11 This article explores the six key points to take away from the consensus statement to guide management of older adults with diabetes and frailty in primary care.
1. Use a Simple Assessment of Frailty
Frailty is often considered as a binary (yes or no) complication of ageing, but in reality it is a continuum of a syndrome. Although there is no consensus definition, frailty reflects a state of increased vulnerability to adverse health outcomes for individuals of the same chronological age. In practice, there must be poor performance in three of five criteria:12
- weight loss
- exhaustion
- weakness (sarcopenia)
- slowness
- lack of functional activity.
Around 10% of people aged more than 65 years are living with frailty,1 which increases at a rate of 3% per year in independent, community-dwelling older adults.13
Two tools are commonly used to assess frailty in clinical practice, the electronic Frailty Index (eFI),9 which is embedded in many electronic patient record systems, and the Rockwood scale.14 The eFI is a ratio of the number of deficits that an individual has accumulated, divided by all the deficits measured. It is dependent on the accuracy of the primary coding. In my experience, it tends to overestimate the degree of frailty in people with diabetes, who are more likely to have been coded for the presence or risk of cardiovascular disease, renal impairment, peripheral vascular disease, and foot care, as well as polypharmacy and dizziness or falls, if they have experienced hypoglycaemia. The Rockwood scale describes functional ability and is the gold standard for research and comprehensive geriatric assessments. However, in day-to-day practice, this detailed assessment adds little to care compared to a simple assessment that places older patients into one of three broad categories:
- fit or mildly frail with few co-existing chronic illnesses and intact cognitive and functional status
- moderately frail individuals who may have multiple comorbidities causing impairment in some activities of daily living (ADLs), although for the most part they are independent
- severely frail individuals who may have multiple other long-term conditions, moderate-to-severe cognitive impairment, and are dependent on others to assist in ADLs.
Although these three broad categories can be readily assessed in a very short consultation by any healthcare worker who knows their patients well, they provide important information about prognosis and, therefore, expected benefit from any intervention. As such, they become the basis for target setting and treatment options. However, frailty is not unidirectional and, in my clinical experience, it can be reversed if underlying conditions are managed.
2. Set Targets to Intensify Pharmacotherapy, and Thresholds to De-escalate Agents
Despite the recommendations of national and international guidelines to individualise care for older adults,6 there is a dearth of evidence about whether this is feasible, let alone whether it benefits patients. To date, only one study has attempted to evaluate setting individualised treatment targets for frail older adults.15 However, despite intensive training in the evaluation of frailty, polypharmacy, and baseline comorbidities and glycated haemoglobin (HbA1c), investigators set a goal for glycaemic control of 53 mmol/mol (7%) in a population aged between 70 and 97 years, aligned to local guidelines for younger adults.15 Therefore, guidance published by a stakeholder group established treatment targets for older adults with diabetes according to their frailty status (Table 1).7 Fit older adults should be treated to a target of 58 mmol/mol (7.5%), the value that would be expected to reduce microvascular and macrovascular complications within their anticipated life expectancy.7
Table 1: Treatment Targets and De-escalation Thresholds Based on Frailty Status of Older Adults with Diabetes7
| Patient Characteristics | Target HbA1c | De-escalation Threshold |
|---|---|---|
| The fit older adult with diabetes | 58 mmol/mol (7.5%) | 53 mmol/mol (7.0%) |
| Moderate to severe frailty | 64 mmol/mol (8.0%) | 58 mmol/mol (7.5%) |
| Very severe frailty | 70 mmol/mol (8.5%) | 64 mmol/mol (8.0%) |
Moderate frailty is associated with a shorter life expectancy, such that even optimum glycaemic control would not be anticipated to reduce macrovascular complications within the time in the treatment range. Less aggressive glycaemic control of approximately 64 mmol/mol (8%), however, would reduce the risk of microvascular complications, such as renal impairment, retinopathy, and dementia.7
For people who are severely frail, their comorbidities would be the main determinant of life expectancy; however, a target of 70 mmol/mol (8.5%) would be expected to improve their quality of life by reducing the risk of infections, candidiasis, and nocturnal enuresis, with an impact upon general cognitive ability.7
For each stage of frailty, de-escalation thresholds have been set, below which there would be little advantage for an individual to be targeted.7 That does not mean that this is a lower target; however, if HbA1c falls below this level, consideration should be given to the potential harm that can be caused by the medications that keep it at that level, with limited gain, and deprescribing can, therefore, occur.
3. Consider Lifestyle Changes that Reflect Frailty Status
Traditionally, lifestyle interventions have been the first stage of diabetes management. The focus of diet and exercise is weight loss to reduce insulin resistance and optimise the residual endogenous insulin production. Paradoxically, frailty is characterised by weight loss, malnutrition, or sarcopenia.16,17 Therefore, any targeted weight loss should be modest at 5–7%, even in the fit or pre-frail older adult with diabetes and obesity.18,19 For those who have progressed to moderate frailty or beyond, the focus of diet and exercise is to maintain weight.18 Optimal nutrition with adequate protein intake of approximately 0.8 g protein/kg is recommended for maintaining muscle volume in such patients.18,19 However, there are potential disadvantages associated with high protein intake—for example, red meat may increase the risk of end-stage renal disease.20
4. Choose Pharmacotherapy Based on Frailty Status
The past 15 years has seen a dramatic increase in the therapeutic options for people living with diabetes. The licensing processes for these agents have gone through major reforms due to the need for cardiovascular safety studies and specific data in common comorbidities, such as renal impairment. Few drugs have been tested specifically in patients with frailty; therefore, most recommendations are based on extrapolation of data from younger populations. Each drug has specific benefits and risks (Table 2).11 These different relative merits of drug classes provide advantages in different comorbidities, complications of diabetes, and, therefore, individuals living with diabetes. However, with greater choice comes greater uncertainty. Navigating the evidence profiles of the different available classes, and then extrapolating these to populations that were not formally evaluated in the trial programme, leads to heterogeneity of implementation. To combat this, the stakeholder group produced three treatment algorithms based on an individual’s frailty status (Figure 1).11
Table 2: The Pros and Cons of Current Non-insulin Antihyperglycaemic Therapies for Older Adults11
| Antihyperglycaemic Therapy | Pros | Cons | |
|---|---|---|---|
| Metformin |
|
| |
| Sulfonylurea |
|
| |
| DPP-4i |
|
| |
| SGLT2i |
|
| |
| GLP-1 RA |
|
| |
| TZD |
|
| |
| |||
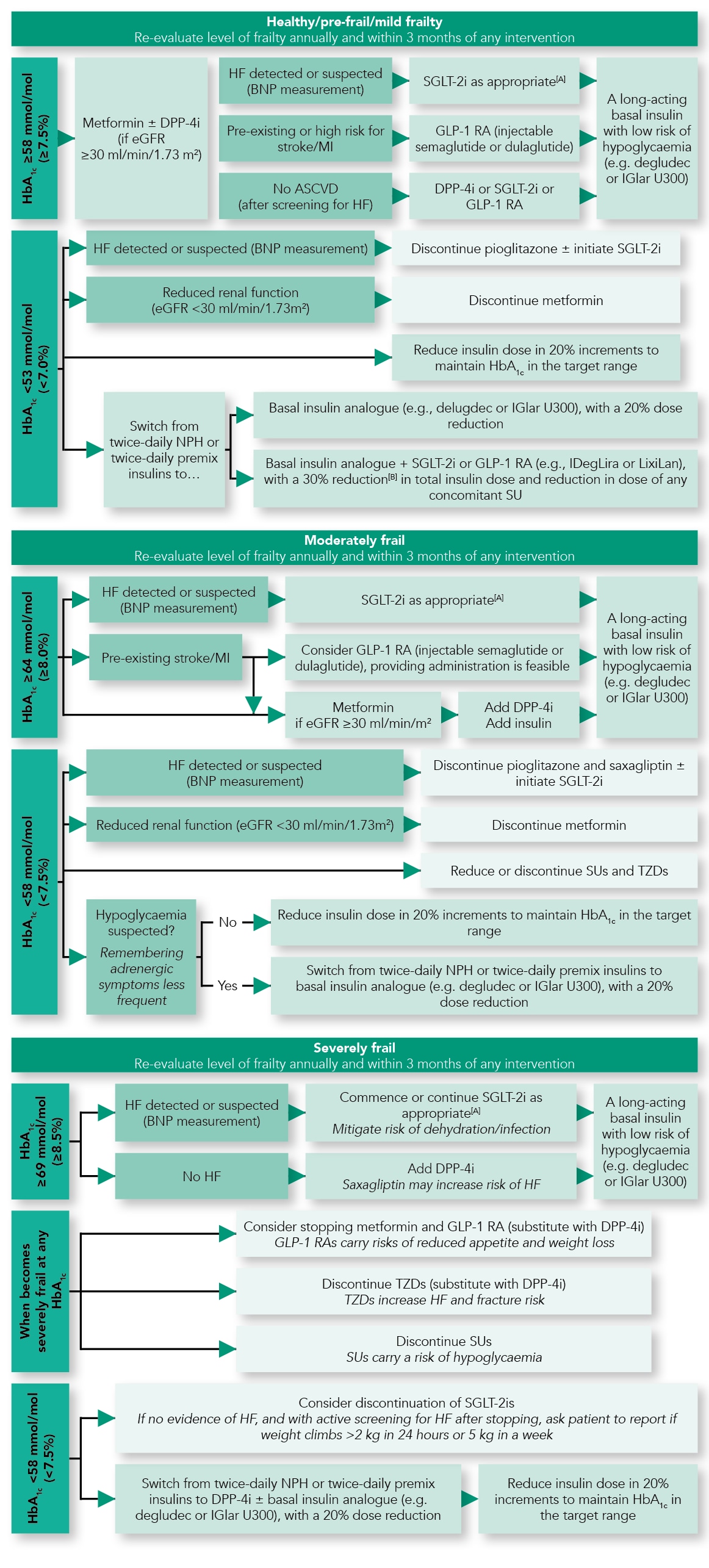
[A] At time of publication, treatment with any SGLT2i can be initiated at eGFR >60 ml/min/1.73 m2 for the management of hyperglycaemia: canagliflozin can be initiated at >45 ml/min/1.73 m2 or >30 ml/min/1.73 m2 in people with proteinuria; dapagliflozin can be initiated at any HbA1c for the management of heart failure. All SGLT2is are less efficacious at reducing hyperglycaemia at lower eGFRs.
[B] Expert recommendation.
HbA1c =glycated haemoglobin; DPP-4i=dipeptidyl peptidase-4 inhibitor; eGFR=estimated glomerular filtration rate; HF=heart failure; BNP=brain natriuretic peptide; MI=myocardial infarction; ASCVD=atherosclerotic cardiovascular disease; SLGT2i=sodium–glucose co-transporter-2 inhibitor; GLP-1 RA=glucagon-like peptide-1 receptor agonst; IGlar U300=insulin glargine 300 units/ml; NPH=neutral protamine Hagedorn; IDegLira=insulin degludec/liraglutide; LixiLan=lixisenatide/insulin glargine; SU=sulfonylurea; TZD=thiazolidinediones
Adapted with permission from Strain WD, Down S, Brown P et al. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabet Ther 2021; 12: 1227–1247.
For healthy older adults, given their anticipated life expectancy, the treatment strategy is based on the existing American Diabetes Association and European Association for the Study of Diabetes guidance.6 For those with moderate frailty, wider consideration should be given to drugs that work on comorbidities, such as stroke, myocardial infarction, or heart failure, which are likely to give benefit within anticipated life expectancy. If there are no relevant comorbidities, the treatment strategy should be one that minimises the risk of hypoglycaemia or other complications of diabetes. People who are severely frail require therapeutics that can achieve relaxed glycaemic control, minimise day-to-day variability, and have a low risk of hypoglycaemia and other complications. In this situation, a long-acting insulin, or a low dose of ultralong-acting insulin, such as insulin degludec or U300 insulin glargine, can achieve glycaemic control with a lower risk of hypoglycaemia.11 Although these insulins are regarded as ‘premium’, even a marginal reduction in hypoglycaemia in a population that is likely to have an increased duration of hospitalisation when admitted with hypoglycaemia may be hugely cost effective.21
5. Provide Practical Tips to Encourage Medication Adherence
For the most part, the introduction of these agents should be approached in the same way as for any other patient. Metformin is associated with nausea and appetite suppression. A simple modification is to initiate a slow-release preparation that causes fewer side effects. After the patient has become used to the drug, they can be switched to standard release, with confidence in their use. Metformin should be commenced at a low dose of 500 mg and escalated as tolerated and necessary, up to 1 g twice daily.22
Dipeptidyl peptidase-4 inhibitors (DPP-4is) are well tolerated and can be commenced at an optimal dose with very few side effects.23 Once established, it is often worth considering fixed-dose combination tablets with metformin to reduce tablet burden. Given the multiple comorbidities of older adults, many will experience polypharmacy, and anything that can be done to improve adherence will benefit patients. DPP-4is work on the same physiological pathway as glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and should therefore not be co-administered.
As heart failure affects in excess of 22% of older adults with diabetes24 and sodium–glucose co-transporter-2 inhibitors (SGLT2is) have proven benefit in a short time, these agents should perhaps be more widely used. When they were introduced, there was concern that they might cause a decline in renal function. The clinical trials, however, showed that after a short-term dip in estimated glomerular filtration rate, certain SGLT2is had renal benefits.25,26 The trials in heart failure required no modification in loop diuretics and did not trigger postural hypotension.25–28 As such, SGLT2is can be commenced without the need to monitor creatinine or modify antihypertensives. The risk of candidiasis has been reported in several studies;25,28 however, this can be minimised with close attention to personal hygiene, and is easily treated with clotrimazole cream.
GLP-1 RAs can be administered daily, once weekly, or now in an oral formulation. As part of their mechanism of action, they cause nausea. To reduce the severity of symptoms, patients can be advised to eat small, frequent meals rather than one large meal. Furthermore, if the patient expects it to occur and knows it is self-limiting they may find it easier to work through this.
6. Evaluate, Reassess, and Deprescribe when Necessary
As with any person living with diabetes, an individual’s therapeutic response may differ based on physiological activity. Every adjustment to medication needs to be evaluated within 3 months of the intervention.11 The evaluation of effect should extend beyond simply assessing the impact on HbA1c levels. An agent that improves HbA1c may improve overall wellbeing and reduce frailty, such that targets should be more aggressive than originally proposed. Alternatively, the introduction of agents that trigger hypoglycaemia may cause a decline in physical function or confidence, and, therefore, result in a de-escalation of treatment to improve overall wellbeing.11
Once a patient’s frailty progresses, their medication requires re-evaluation to reduce the potential for complications.11 Deprescribing is an active process requiring multiple steps. All prescribed medicines should be considered to identify risks compared to potential benefits. These need to be evaluated in the context of the individual patient’s overall health goals.11 The most difficult element, however, is communicating these decisions to the individual. After decades of reinforcing the importance of regular medication adherence, good glycaemic control, and tight blood pressure targets, it can be disconcerting to any person with diabetes to be told that medications are being discontinued. It may be perceived as rationing or cost-cutting, or that the healthcare system has given up hope for the individual and simply wishes to expedite their death.11 Focus on the potential harm of agents and the time window to benefit, which may include candid conversations about the mortality of the individual. Often the compromise is that medications are held for a defined period, with an agreement in place for review and measures that will be taken in the event of symptoms recurring. As with treatment escalation, the impact of deprescribing should be evaluated at a minimum of 3 months after the intervention.11
Summary
As older adults with diabetes become frail, the priority for treatment is to improve quality of life rather than prolong it. This includes adjusting the criteria by which interventions are chosen from long-term risk to tolerability and safety. De-escalation of drugs also plays a major role in reducing the risk of iatrogenic complications. This approach, however, requires working together with older adults living with diabetes and their families and carers to achieve the goal of putting life in the remaining years.
Dr David Strain
Senior Clinical Lecturer, University of Exeter Medical School
| Key Points |
|---|
SGLT2i=sodium–glucose co-transporter-2 inhibitor; GLP-1 RAs=glucagon-like peptide-1 receptor agonists; DPP-4i=dipeptidyl peptidase-4 inhibitor |
| Implementation Actions for STPs and ICSs |
|---|
Written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved in implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system |

