Dr Pam Brown and Dr Colin Kenny Discuss Recommendations From a New International Guideline on Diabetes Management in Patients with Chronic Kidney Disease
| Read This Article to Learn More About: |
|---|
Find COVID-19 considerations and implementation actions for STPs and ICSs at the end of this article |
The first Kidney Disease: Improving Global Outcomes (KDIGO) guideline on diabetes management in chronic kidney disease (CKD) was published in October 2020. The aims of the guideline are to: provide evidence-based recommendations for the care of people with diabetes and CKD; integrate new data with existing evidence; and discuss practical implementation and health systems approaches to managing diabetes in people with CKD. The guideline has been developed at a time of significant changes to guidance on the management of CKD and helps to highlight these changes and the evidence base underpinning them.1
The new guideline is the first of the KDIGO guidelines to be presented in the new format. Previous KDIGO guidelines have included recommendations and ‘ungraded statements’. The new format continues to include recommendations designed to be actioned, which are made when there is a systematic review and clear evidence that one option is better than another. In the full document, each recommendation is supported by one or two sentences summarising what was considered when making the recommendation, discussion of the balance of benefits and harms, the supporting evidence base, and the rationale behind the recommendation. ‘Practice points’ represent the consensus of the international work group (guideline development group), and are made when there is no systematic review or the evidence is less robust yet the work group felt that clinicians need guidance and would value expert opinion. In the new format, practice points include tables, figures, and algorithms as well as text. Unlike recommendations, these are discretionary, and it is the clinician’s decision whether to implement them.1 In several sections of the guideline, the work group decided that there was no new evidence necessitating a change in practice in relation to CKD and type 1 diabetes; therefore, they did not duplicate existing advice, but recommended that other guidelines continue to be followed instead.1
In this article, we share the recommendations and practice points of the new KDIGO guideline on diabetes management in CKD and discuss the similarities and differences between the KDIGO guideline and other guidelines in current use in the UK (the American Diabetes Association [ADA]/European Association for the Study of Diabetes [EASD] 2018 consensus on glycaemic management of type 2 diabetes and its 2019 update,2,3 the European Society of Cardiology [ESC]/EASD guideline,4 the Scottish Intercollegiate Guidelines Network [SIGN] guideline,5 and the Primary Care Diabetes Europe [PCDE] guideline6). We also offer suggestions on how we, in primary care, can use this guideline as a benchmark to improve our management of people with CKD and type 2 diabetes.
Why is the KDIGO Guideline Useful?
As was recently highlighted in relation to the ADA/EASD and ESC guidelines,7 it is important that the existence of multiple different diabetes guidelines does not lead to confusion and clinical inertia. Evidence-based management of type 2 diabetes and its complications is increasingly complex; therefore, it is important that guidelines simplify, rather than further complicate, treatment choices. NICE Guideline 28, Type 2 diabetes in adults: management, is out of date because it does not take into account recent cardiovascular outcome trials (CVOTs);8 similarly, the 2017 update to SIGN 154, Pharmacological management of glycaemic control in people with type 2 diabetes, is partially out of date in this respect.5 However, comparisons show that there are more similarities than differences between the up-to-date guidelines in common use—all recommend sodium–glucose co-transporter-2 inhibitors (SGLT2is) for slowing the progression of CKD and improving cardiovascular disease (CVD) outcomes in people with type 2 diabetes and CKD.
When managing people with type 2 diabetes, decisions revolve around:
- whether to start with monotherapy or combination therapy
- whether the added benefit of a glucagon-like peptide-1 receptor agonist (GLP-1 RA) for atherosclerotic cardiovascular disease, or SGLT2is for heart failure (HF) or renal disease, is needed immediately
- how to achieve and maintain agreed glycated haemoglobin (HbA1c) targets to minimise exposure to hyperglycaemia.
The KDIGO guideline (along with the KDIGO guidelines on the management of blood pressure [BP; currently being updated]9 and lipids10 in CKD) provides a clear pathway for managing people with CKD and diabetes. The recommendation to start treatment with a combination of metformin and an SGLT2i, provided that estimated glomerular filtration rate (eGFR) is ≥30 ml/min/1.73 m2, is a significant variation from some, but not all, other guidelines in common use.1
The guideline and its supporting documents are published on the MAGICapp web platform, which allows readers to access evidence directly linked to the recommendations:1
The document uses conventional rather than système international units for creatinine and glucose, Diabetes Control and Complications Trial (%) rather than International Federation of Clinical Chemistry units (mmol/mol) for HbA1c, and mg/g rather than mg/mmol units for albumin:creatinine ratio (ACR). In this article, the units have been converted into units commonly used in the UK.
CKD and Diabetes
KDIGO defines CKD as ‘abnormalities of kidney structure or function, present for >3 months, with implications for health’. CKD is classified based on cause, glomerular filtration rate category (G1–G5), and albuminuria category (A1–A3). The relative prognosis—green for low risk; yellow for moderately increased risk; orange for high risk; and red for very high risk—is shown in Figure 1.1
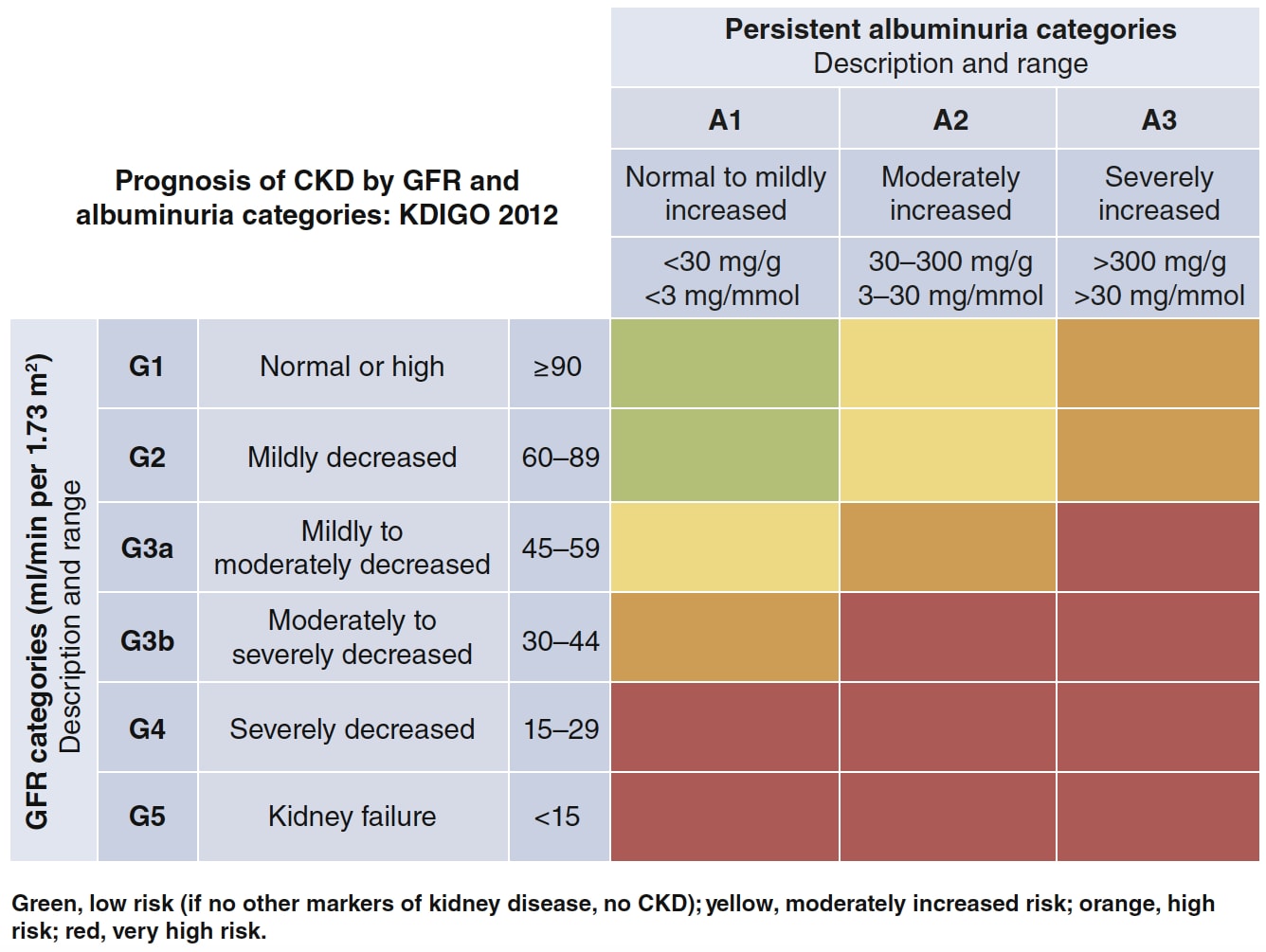
CKD=chronic kidney disease; GFR=glomerular filtration rate; KDIGO=Kidney Disease: Improving Global Outcomes
Kidney Disease: Improving Global Outcomes. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98 (4): S1–S115. Reproduced with permission
Confusion surrounds the relationship between CKD and diabetic kidney disease (DKD). DKD, or diabetic nephropathy, is defined as hyperglycaemia-induced glomerular disease that results in gradually rising urine ACR followed by a gradual decline in eGFR. Usually, in people with diabetes, if there is no other obvious cause for the decline in renal function, it can be attributed to diabetes, especially if other microvascular complications such as retinopathy are present; in these circumstances, DKD and CKD are used interchangeably. However, hypertension and other conditions can damage the glomerulus or cause tubular or interstitial fibrosis and result in CKD in people with or without diabetes, so if no retinopathy is present, further investigation for an underlying cause may be appropriate.11
It is an important time to focus on CKD and type 2 diabetes from a primary care perspective because CVOTs of newer drugs suggest that they may slow CKD progression. Recently, CVOTs involving populations with type 2 diabetes and CKD (CREDENCE12 and DAPA-CKD13) have confirmed the benefits of specific glucose-lowering drugs in slowing the rate of progression of CKD as well as reducing the risk of poor CVD outcomes. However, to optimise these benefits, primary care teams need to diagnose CKD early, ideally when only low levels of albuminuria are present, so that they can immediately take action to decrease CKD progression, reduce the risk of CVD, and ultimately decrease mortality in this high-risk group.
People with type 2 diabetes are at more than twice the risk of myocardial infarction than those without diabetes.14 Around 40% of people with type 2 diabetes have some form of CKD, and the combination of diabetes and CKD greatly increases risk of CVD and mortality compared with diabetes alone.14–18 There is a tenfold estimated increased absolute risk of mortality in those with type 2 diabetes, albuminuria, and impaired eGFR compared with those with type 2 diabetes and no kidney disease,18 rising from a 4.1% 10-year cumulative mortality with type 2 diabetes but no CKD to a 47% mortality in those with type 2 diabetes and both albuminuria and impaired eGFR. The mortality risk increases fourfold once albuminuria intervenes.18
The KDIGO guideline is also important from a primary care perspective because there remains a gap between evidence-based care and current practice in this area.15,19 Clinical trial evidence published in the last few years also significantly strengthens the role for SGLT2is and GLP-1 RAs in slowing renal decline and reducing the risk of CVD.12,13 Recent guidelines (such as the ADA/EASD consensus report on glycaemic management2,3 and the PCDE guideline6) recommend changes in practice based on the new evidence. Previously in the UK, SGLT2is could only be initiated in patients with an eGFR ≥60 ml/min/1.73 m2, although they could be continued until eGFR is persistently <45 ml/min/1.73 m2, meaning that only people with albuminuria and preserved eGFR could benefit from their initiation. A change in licensing now permits the initiation of at least one SGLT2i in people with CKD,20 and it is likely that additional drugs in this class will gain licence extensions to bring them in line with licences in other parts of the world.
Practice Points and Recommendations on Type 2 Diabetes and CKD
Key goals for primary care teams in managing people with type 2 diabetes and CKD are shown in Figure 2. An overview of the recommendations in the guideline regarding kidney–heart risk factor management are shown in Figure 3.1
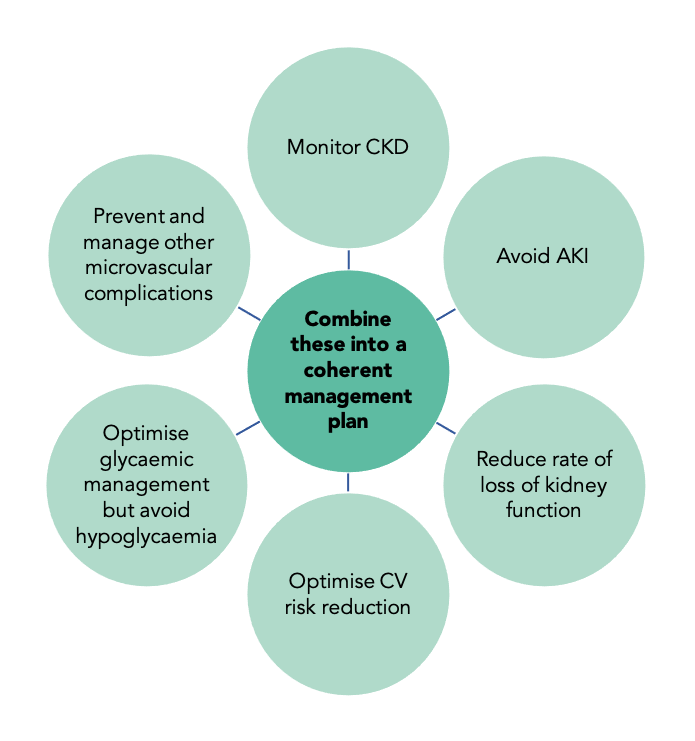
CKD=chronic kidney disease; AKI=acute kidney injury; CV=cardiovascular
© Dr Pam Brown and Jane Diggle
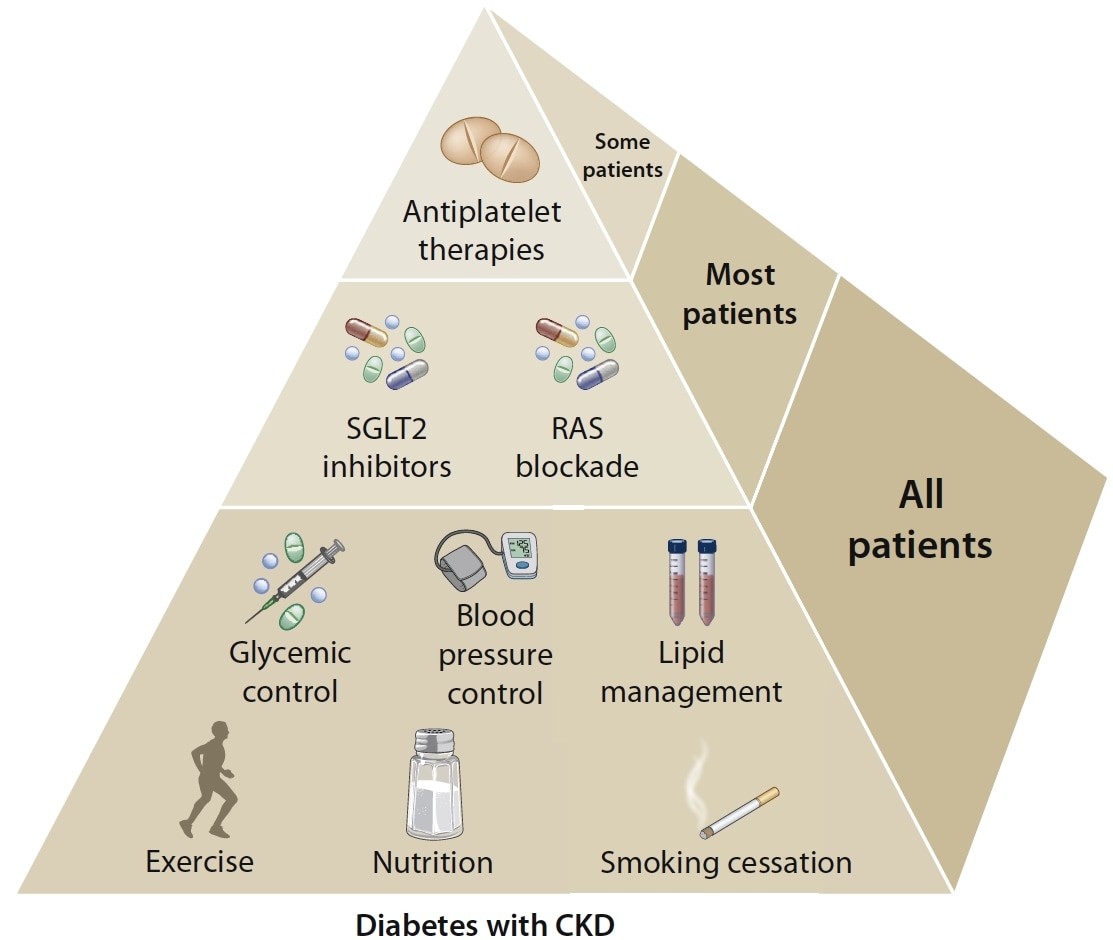
Glycaemic control is based on insulin for type 1 diabetes and a combination of metformin and SGLT2is for type 2 diabetes, when eGFR is ≥30 ml/min/1.73 m2. SGLT2is are recommended for patients with type 2 diabetes and CKD. RAS inhibition is recommended for patients with type 1 or type 2 diabetes with albuminuria and hypertension. Aspirin generally should be used lifelong for secondary prevention among those with established cardiovascular disease and may be considered for primary prevention among high-risk individuals, with dual antiplatelet therapy used in patients after acute coronary syndrome or percutaneous coronary intervention.
SGLT2i=sodium–glucose co-transporter-2 inhibitor; RAS=renin–angiotensin system; CKD=chronic kidney disease; eGFR=estimated glomerular filtration rate
Kidney Disease: Improving Global Outcomes. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98 (4): S1–S115. Reproduced with permission
The guideline does not cover prevention, diagnosis, or monitoring of CKD, which are covered by other documents, including the UK guideline Testing for kidney disease in type 2 diabetes: consensus statement and recommendations.11
The practice points and recommendations of the KDIGO guideline on the management of diabetes and CKD are summarised in Table 1. Note: the recommendations and practice points listed in Table 1 are specifically for individuals with diabetes and CKD, and only points with which readers may be less familiar have been included; for the complete recommendations, see the full guideline.1
Table 1: Summary of Practice Points and Recommendations for Diabetes and CKD1
| Practice Points and Recommendations | Action |
|---|---|
| Comprehensive Care | |
| Practice point 1.1.1 | Treat with a comprehensive strategy to reduce risks of CKD progression and CVD |
| Recommendation 1.2.1 | Treat patients with diabetes, hypertension, and albuminuria with an ACEI or ARB; titrate to the highest approved dose tolerated |
| Practice point 1.2.1 | In patients with diabetes, albuminuria, and normal BP, consider an ACEI/ARB |
| Practice point 1.2.2 | Monitor BP, serum creatinine, and potassium within 2–4 weeks of ACEI/ARB initiation or dose increase |
| Practice point 1.2.3 | Continue ACEI/ARB therapy unless serum creatinine increases by more than 30% within 4 weeks of initiation or dose increase |
| Practice point 1.2.4 | Advise contraception in women receiving an ACEI/ARB; discontinue when pregnant or considering pregnancy |
| Practice points 1.2.5 and 1.2.6 | Manage hyperkalaemia associated with ACEI/ARB use |
| Practice point 1.2.7 | Use only one agent at a time to block RAS—do not use an ACEI and an ARB in combination or with a direct renin inhibitor |
| Practice point 1.2.8 | Mineralocorticoid receptor antagonists are effective for refractory hypertension, but may cause hyperkalaemia or reversible decline in GFR |
| Recommendation 1.3.1 | Advise people who use tobacco products to quit |
| Practice point 1.3.1 | Counsel patients to reduce second-hand smoke exposure |
| Glycaemic Monitoring and Targets | |
| Recommendation 2.1.1 | Use HbA1c to monitor glycaemic control |
| Practice point 2.1.1 | Measure HbA1c twice per year; four times per year if target not met or change in therapy |
| Practice point 2.1.2 | HbA1c is less accurate with advanced CKD (G4–G5) and has low reliability in those on dialysis |
| Practice point 2.1.3 | Consider use of a GMI from CGM data if HbA1c does not match SMBG or symptoms |
| Practice point 2.1.4 | Daily CGM or SMBG may help prevent hypoglycaemia and should be considered when therapies increase risk (insulin, SUs) |
| Practice point 2.1.5 | Increased hypoglycaemia risk in those with advanced CKD—avoid SUs or insulin when possible in the absence of SMBG/CGM |
| Recommendation 2.2.1 | Set an individualised HbA1c target of <6.5% to <8.0% (<48 to <64 mmol/mol) in patients not on dialysis |
| Practice point 2.2.1 | Safe achievement of low HbA1c targets (<6.5% or 7.0%, <48–53 mmol/mol) may be facilitated by CGM or SMBG and using drugs not associated with hypoglycaemia |
| Practice point 2.2.2 | CGM metrics such as time in range and time in hypoglycaemia may be alternatives to HbA1c for some people |
| Lifestyle Interventions—Nutrition | |
| Practice point 3.1.1 | Recommend an individualised diet high in vegetables, fruits, wholegrains, fibre, legumes, plant-based proteins, unsaturated fats, and nuts; and low in processed meats, refined carbohydrates, and sweetened beverages |
| Recommendation 3.1.1 | If not on dialysis, maintain a protein intake of 0.8 g/kg body weight per day |
| Practice point 3.1.2 | If on haemodialysis and particularly peritoneal dialysis, recommend consumption of 1–1.2 g protein/kg body weight per day |
| Recommendation 3.1.2 | Keep sodium intake <2 g per day (<90 mmol sodium/day; <5 g sodium chloride/day) |
| Practice point 3.1.3 | Shared decision-making should be a cornerstone of patient-centred nutrition management |
| Practice point 3.1.4 | Involve experts on the provision of nutritional advice in the multidisciplinary team |
| Practice point 3.1.5 | Consider cultural differences, food intolerances, variations in food resources, cooking skills, co-morbidities, and cost when recommending dietary options |
| Lifestyle Interventions—Physical Activity | |
| Recommendation 3.2.1 | Advise moderate-intensity PA for at least 150 minutes/week or to a level compatible with their CV and physical tolerance |
| Practice point 3.2.1 | Recommendations for PA should consider age, ethnicity, presence of other co-morbidities, and access to resources |
| Practice point 3.2.2 | Avoid sedentary behaviour |
| Practice point 3.2.3 | If at higher risk of falls, provide guidance on intensity and type of PA |
| Practice point 3.2.4 | Consider advising/encouraging people with obesity to lose weight, particularly people with an eGFR ≥30 ml/min/1.73 m2 |
| Antihyperglycaemic Therapies (only recommendations are listed here and the practice points including algorithms are discussed in the text) | |
| Recommendation 4.1.1 | Metformin is recommended if eGFR ≥30 ml/min/1.73 m2 |
| Recommendation 4.2.1 | An SGLT2i is recommended if eGFR ≥30 ml/min/1.73 m2 |
| Recommendation 4.3.1 | If the individualised glycaemic target is not achieved with metformin and a SGLT2i or in those who are unable to use these medications, a long-acting GLP-1 RA is recommended |
| Approaches to Management | |
| Recommendation 5.1.1 | Structured self-management educational programme recommended |
| Recommendation 5.2.1 | Policymakers and institutional decision-makers should implement team-based integrated care, focused on risk evaluation and patient empowerment, to provide comprehensive care |
| Recommendations are graded by whether a given course of action is recommended (level 1) or suggested (level 2), and by the quality of evidence (A=high, B=moderate, C=low, D=very low). See p.S7 of the full guideline for a more detailed expansion of the grading system. | |
| CKD=chronic kidney disease; CVD=cardiovascular disease; ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; BP=blood pressure; RAS=renin–angiotensin system; GFR=glomerular filtration rate; HbA1c =glycated haemoglobin; GMI=glucose management indicator; CGM=continuous glucose monitoring; SMBG=self-monitoring of blood glucose; SU=sulfonylurea; PA=physical activity; CV=cardiovascular; eGFR=estimated glomerular filtration rate; SGLT2i=sodium–glucose co-transporter-2 inhibitor; GLP-1 RA=glucagon-like peptide-1 receptor agonist. | |
| Note: the recommendations and practice points listed in this table are specifically for individuals with diabetes and CKD, and only points with which readers may be less familiar have been included; for the complete recommendations, see the full guideline. Adapted from Kidney Disease: Improving Global Outcomes. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98 (4): S1–S115. | |
Several of the practice points in the full guideline are supported by algorithms, figures, and tables that are useful for primary care teams. The guideline contains a table of the primary endpoints and renal outcomes of the CVOTs with SGLT2is, GLP-1 RAs, and dipeptidyl peptidase-4 (DPP-4) inhibitors that supports decision-making (see p.S59 of the full guideline).1
Comprehensive Care
People with diabetes and CKD are at high risk of acute complications of diabetes, such as hypoglycaemia and diabetic ketoacidosis, long-term CKD progression requiring dialysis and transplantation, other microvascular complications, and CVD such as ischaemia, arrhythmias, and HF.1 Management is complex and requires multidisciplinary team input across primary and secondary care. Goals should include management of hypertension, lipids, and glycaemic control, and tackling risk factors for both CVD and CKD progression, including smoking cessation. Management of hypertension,9 lipids,10 and other aspects of advanced CKD such as dialysis, transplantation, bone disease, and anaemia are covered by other KDIGO guidelines.21–24 Glycaemic management with insulin for type 1 diabetes is not discussed in this guideline.
Although renin–angiotensin system (RAS) blockade is recommended for people with type 1 or type 2 diabetes, hypertension, and albuminuria (persistent ACR ≥3 mg/mmol) to reduce CKD progression, an effect that may be independent of BP, the evidence for reduced progression is less clear in those without albuminuria or without hypertension. In the absence of albuminuria, people with diabetes and hypertension are at lower risk of CKD progression, and evidence of a benefit in terms of slowing CKD progression is less clear. Likewise, data are scarce on the effects of RAS inhibition in those with diabetes and albuminuria but normal BP. For this reason, the guideline only recommends that RAS inhibition be considered in those with diabetes if BP is normal and in those without albuminuria. A combination of an angiotensin-converting enzyme inhibitor (ACEI) and an angiotensin receptor blocker (ARB) or direct renin inhibitor may be harmful and should not be used.1
The ACEI (or ARB) should be titrated to the maximum recommended or tolerated dose with careful monitoring of potassium and renal function. Hyperkalaemia, or a >30% increase in creatinine within 4 weeks of initiation or dose increase, should prompt primary care teams to reduce the dose or stop the drug and seek guidance. Women of childbearing age must use reliable contraception, and these drugs should be stopped when pregnancy is planned.1
Although mineralocorticoid receptor antagonists may be helpful for reducing BP, they can cause hyperkalaemia or a reversible decline in renal function, particularly among patients with a low eGFR, so they should be used with care.1
Advise smoking cessation in all people with diabetes and CKD and consider advice to reduce exposure to second-hand smoke. Aspirin should be recommended for secondary prevention of CVD and, in some people at very high risk, for primary prevention also. See Figure 3 for a summary of kidney–heart risk factor management.1
Glycaemic Monitoring and Targets
The guideline recommends that HbA1c is used for monitoring, and that an individualised HbA1c goal of <6.5 to <8.0% (<48 to <64 mmol/mol) is agreed depending on risk factors (see Figure 9, p.S24 of the full guideline).1
Glycaemic targets are set to prevent complications and avoid hypoglycaemia. In randomised controlled trials, lower HbA1c has been shown to reduce the risk of microvascular complications (such as kidney disease, retinopathy, and neuropathy), and in some studies, reduce macrovascular complications (CVD events).1
HbA1c is an advanced glycation end product of haemoglobin, reflecting glycaemia over the red blood cell lifespan (8–12 weeks) and correlating moderately with other measures of glucose in those with both type 1 or 2 diabetes and CKD, except in patients with advanced CKD receiving dialysis. HbA1c is the measurement of choice for long-term glycaemic monitoring in CKD. In those for whom HbA1c monitoring is unsuitable, glycated albumin and fructosamine have been proposed for long-term monitoring and reflect a shorter 2–4-week timeframe. Glycated albumin is associated with all-cause and cardiovascular mortality in those on chronic haemodialysis. Both glycated albumin and fructosamine are affected by hypoalbuminaemia, which is common in those with CKD, and neither appear to have advantages over HbA1c. In advanced CKD, especially in patients receiving dialysis, HbA1c is likely to be lowered by the shorter lifespan of erythrocytes due to anaemia, transfusion, erythropoietin therapy, or iron replacement therapies, and increased by advanced glycation end products caused by processes other than hyperglycaemia (such as inflammation). Measurement twice per year is recommended, with measurement more often if there are changes in lifestyle, glucose-lowering therapy, or fluctuating control.1
Self-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM) are not affected by CKD, including dialysis or transplantation, so consider referral for CGM if SMBG or symptoms do not correlate with measured HbA1c in advanced CKD.1
Individualised glycaemic targets are recommended, and Figure 9 in the full guideline assists in choosing targets based on risk factors (see Figure 9, p.S24 of the full guideline).1 In younger people with milder CKD who are treated with medication that does not increase hypoglycaemia, targets for HbA1c as low as <6.5% or 7% (48–53 mmol/mol) are appropriate, whereas in older, more frail people who have advanced CKD or are treated with insulin or a sulfonylurea (SU), a target of <8% (64 mmol/mol) may be more appropriate. However, newer drugs can achieve tighter control without increased risk of hypoglycaemia.1
Lifestyle Interventions
The KDIGO guideline recommends a protein intake of 0.8 g/kg/day for those with diabetes and CKD not on dialysis, increasing to 1–1.2 g/kg/day in those on dialysis, particularly those on peritoneal dialysis; this may feel counterintuitive, as people with severe CKD are generally encouraged to reduce protein intake. The guideline suggests culturally diverse plate options, each including 50% vegetables and fruit, 25% protein, and 25% wholegrains and starchy vegetables. The guideline offers useful details on the protein content of foods as shown in Table 2.1
Table 2: Protein Content of Common Foods1
| Protein Source | Protein Content |
|---|---|
| Animal proteins | |
| Meat, poultry, fish, seafood | 28 g food=6–8 g protein |
| 1 egg | 6–8 g protein |
| Dairy, milk, yoghurt, cheese | 250 ml=8–10 g protein 28 g cheese=6–8 g |
| Plant proteins | |
| Legumes, dried beans, nuts, seeds | 100 g (0.5 cup) cooked=7–10 g protein |
| Wholegrains, cereals | 100 g (0.5 cup) cooked=3–6 g protein |
| Starchy vegetables, breads | 2–4 g protein |
| Kidney Disease: Improving Global Outcomes. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98 (4): S1–S115. Reproduced with permission | |
The full guideline stipulates that total sodium intake should be kept to <2 g/day (<5 g of sodium chloride per day), and recommends 10 ways to cut out salt, including buying and cooking fresh food, avoiding foods with >400 mg sodium per serving, choosing lower salt alternatives, and using unsalted butter. Because of hyperkalaemia risk, low salt substitutes should be avoided because these are often potassium based.1
Glycaemic Management
An algorithm that guides treatment selection for people with type 2 diabetes and CKD is shown in Figure 4. Recommendations 4.1.1 and 4.2.1 suggest that people with CKD with an eGFR ≥30 ml/min/1.73 m2 and type 2 diabetes will benefit from initiation of metformin and an SGLT2i first line after lifestyle therapy.1 However, this does not currently match licences for SGLT2is in the UK; at present (January 2021), only one drug, canagliflozin, is licensed for initiation in patients with an eGFR <60 ml/min/1.73 m2 to slow renal disease progression.20 Dapagliflozin can be used in people with an eGFR ≥30 ml/min/1.73 m2 but only for HF—so in this context, it can only be used in patients with both HF and CKD.25 At lower eGFR levels, SGLT2is are being used for renal benefit and there may be little glucose lowering, so additional glucose lowering medication will likely be needed.
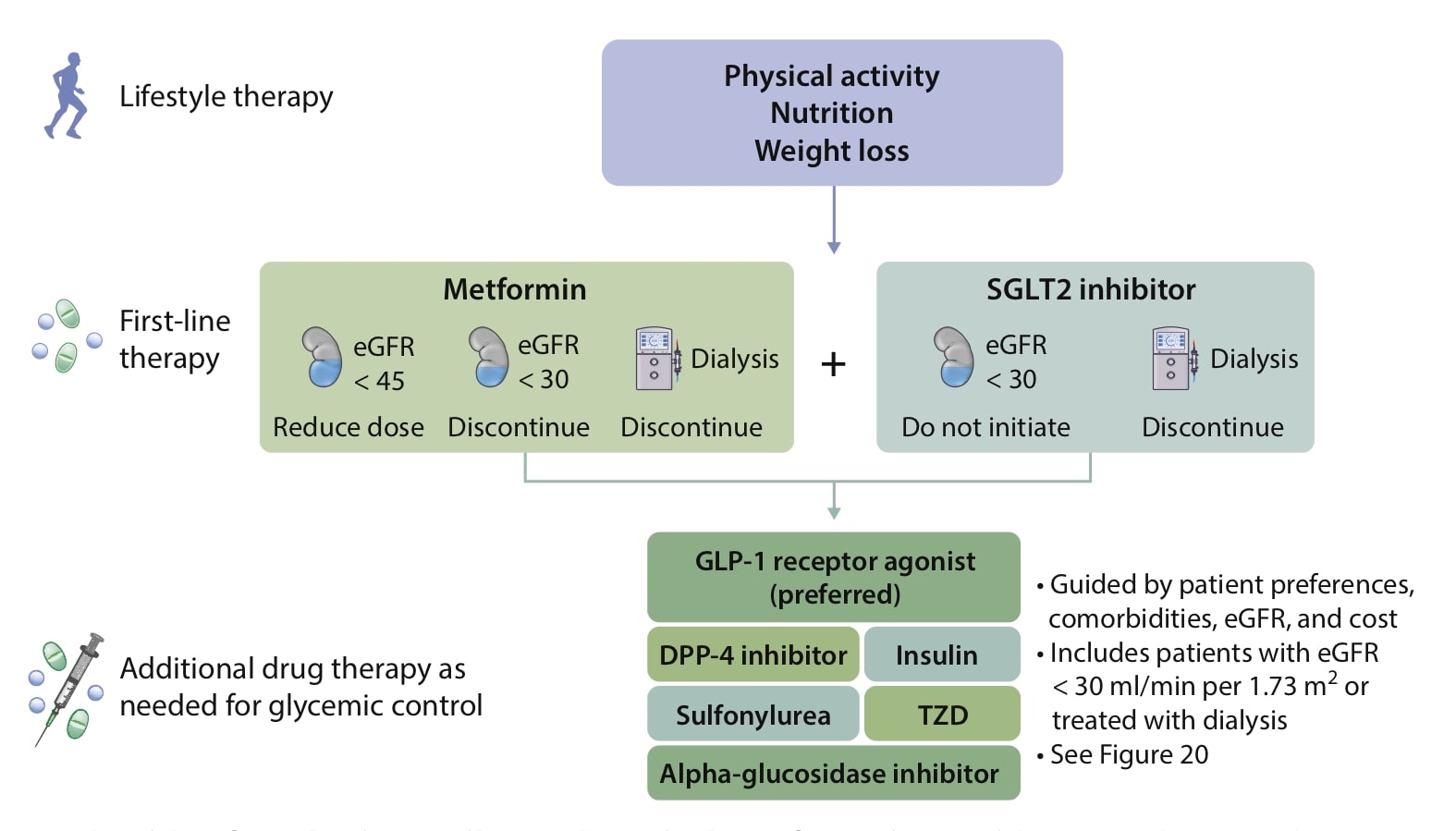
Kidney icon indicates eGFR (ml/min/1.73 m2); dialysis machine icon indicates dialysis.
CKD=chronic kidney disease eGFR=estimated glomerular filtration rate; DPP-4=dipeptidyl peptidase-4; GLP-1=glucagon-like peptide-1; SGLT2=sodium–glucose co-transporter-2; T2D=type 2 diabetes; TZD=thiazolidinedione
Kidney Disease: Improving Global Outcomes. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98 (4): S1–S115. Reproduced with permission
Practice points outline how to initiate metformin, the need to monitor renal function more frequently (two to four times per year) once eGFR is <60 ml/min/1.73 m2, and the necessity of reducing metformin daily dose from 2 g per day to 1 g daily when eGFR drops below 45 ml/min/1.73 m2 and stopping the drug if eGFR is <30 ml/min/1.73 m2, which reflects current practice. The guideline also recommends that patient preferences, co-morbidities, eGFR, and cost should guide the selection of additional drugs to manage glycaemia, when needed, with a GLP-1 RA considered the preferred option. Prescribers are encouraged to measure vitamin B12 levels annually in those on metformin for more than 4 years or at high risk of deficiency. Metformin can be used in renal transplant patients provided that their eGFR is ≥30 ml/min/1.73 m2.1
Practice point 4.2.3 encourages a choice of SGLT2i with documented kidney or cardiovascular (CV) benefits, and recommends that eGFR should be taken into account.1 At the time of writing (January 2021), in the UK, only canagliflozin 100 mg can be initiated at an eGFR of between 45 and 60 ml/min/m2 unless there is significant albuminuria, in which case it can be initiated down to an eGFR of 30 ml/min/1.73 m2.20 This differs from the international recommendations offered in the KDIGO guideline.
Prescribers are reminded that a reversible decrease in eGFR usually occurs in the first few weeks of treatment with SGLT2is, but that this should not prompt cessation of the drug. Unlike with an ACEI and ARB, SGLT2i initiation or titration does not require additional renal monitoring in addition to that based on the baseline eGFR.20 Practice points in the KDIGO guideline encourage withholding SGLT2is during fasting, surgery, or critical illness because of the small risk of diabetic ketoacidosis. In those at risk for hypovolemia, reducing the dose of diuretics should be considered; people should be warned to report symptoms of volume depletion and hypotension, and this should be reviewed as appropriate.1
The guideline suggests that, once SGLT2is are started, they can be continued despite an eGFR <30 ml/min/1.73 m2 until dialysis or transplantation is required.1 However, this does not reflect the licensing situation in the UK, and readers should consult the summary of product characteristics (SmPC) for each individual medication for up-to-date guidance on initiation and continuing therapy.
Because of their ability to slow renal progression, SGLT2is are also recommended as an add-on to other therapies to tighten control or as a switch. It may be necessary initially to reduce the dose of SU or insulin when adding an SGLT2i to avoid hypoglycaemia.1
For those not able to continue on metformin or an SGLT2i, use of GLP-1 RAs with CV benefits is encouraged, and GLP-1 RAs are also recommended alongside SGLT2is for additional glucose lowering.1 The guideline recommends different initiation doses compared with UK guidance; for instance, injectable semaglutide 0.5 mg is recommended for initiation rather than 0.25 mg as recommended in the UK SmPC,26 and dulaglutide 0.75 mg once weekly is recommended, rather than 1.5 mg as recommended in the UK, when initiated in addition to other therapy.1,27 GLP-1 RAs and DPP-4 inhibitors should not be used at the same time, and a reduction in the dose of SU or insulin should be considered to avoid hypoglycaemia when initiating GLP-1 RAs.1
Approaches to Management
The final section of the guideline includes a detailed summary of the evidence on the effects of self-management education on BP, eGFR, HbA1c, and quality of life. The value of multidisciplinary team input, and what the different components of the chronic care model add, are also discussed in relation to CKD and diabetes.1
Comparison with Other Guidelines and Consensus Documents
Box 1 shows a summary of the similarities and differences between the KDIGO guideline and other commonly followed guidelines and consensus documents. These similarities and differences are compared in more detail in Table 3.
| Box 1: Similarities and Differences Between the KDIGO Guideline and Other Guidelines |
|---|
Features of the KDIGO guideline:
How does this differ from other guidelines?
KDIGO=Kidney Disease: Improving Global Outcomes; SGLT2i=sodium–glucose co-transporter-2 inhibitor; CKD=chronic kidney disease; RAS=renin–angiotensin system; eGFR=estimated glomerular filtration rate |
It is important to flag similarities between this and other guidelines to simplify implementation and avoid the risk of clinical inertia. This is particularly likely to be a challenge because licence differences currently mean that, in the UK, only one SGLT2i can be initiated at eGFR <60 ml/min/1.73 m2 to slow renal progression. Also, only one SGLT2i has a licensed indication for use in those with HF, despite there being evidence from clinical trials with other agents.25,28
Table 3: Comparison Table of Different Guidelines—ADA/EASD,2,3 ESC/EASD,4 SIGN,5 PCDE,6 and KDIGO11
| ADA/EASD2,3 | ESC/EASD4 | SIGN5 | PCDE6 | KDIGO1 | |
|---|---|---|---|---|---|
| Date of publication | December 2018; update February 2020 | August 2019 | November 2017 | June 2020 | October 2020 |
| Target audience | Europe/USA | Europe | Scotland/UK | Europe | Global |
| Guideline target | Glycaemic control in type 2 diabetes | Multifactorial management of NDH, type 2 diabetes, and CVD | Pharmacological management of glycaemic control in people with type 2 diabetes | Type 2 diabetes | Type 2 diabetes and CKD |
| Multifactorial risk reduction/targeting | Glycaemic control only | Yes | In full guideline | Yes | Covered by other KDIGO guidelines |
| CV risk categories | ASCVD/high risk of ASCVD; HF or renal disease; none of these | Established CVD; very high, high, and moderate CVD risk | With or without established CV disease | Very high or high CVD risk | Focus on levels of CKD not CVD; those with diabetes mellitus and CKD identified as at very high CVD risk |
| First-line drug therapy | Metformin for all; consider adding GLP-1 RA if ASCVD or high risk | If high/very high CV risk, SGLT2i or GLP-1 RA first line; metformin for added control; metformin first-line only in moderate CV risk | Metformin | Consider metformin AND SGLT2i/GLP-1 RA with CV benefits if very high CV risk; consider metformin and SGLT2i/GLP-1 RA/DPP-4i for high CV risk | Metformin AND SGLT2i if type 2 diabetes and CKD |
| Distinguishing features | Ongoing updating within ADA Standards of Care document when new evidence | Use drugs with CV benefit before metformin in high CV risk groups | Updated to include some earlier CVOTs | Novel risk stratification approach; only two CV risk categories—high and very high CVD risk | Specifically targets type 2 diabetes and CKD |
| ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes; ESC=European Society of Cardiology; PCDE=Primary Care Diabetes Europe; SIGN=Scottish Intercollegiate Guidelines Network; KDIGO=Kidney Disease: Improving Global Outcomes; NDH=non-diabetic hyperglycaemia; CVD=cardiovascular disease; CKD=chronic kidney disease; ASCVD=atherosclerotic cardiovascular disease; HF=heart failure; CV=cardiovascular; GLP-1 RA=glucagon-like peptide-1 receptor agonist; SGLT2i=sodium–glucose co-transporter-2 inhibitor; DPP-4i=dipeptidyl peptidase-4 inhibitor; CVOT=cardiovascular outcome trial. | |||||
How Well are we Managing Type 2 Diabetes in CKD?
It is hoped this guideline will provide a blueprint for early diagnosis and comprehensive, up-to-date management of people with diabetes and CKD.1 Early diagnosis will hopefully lead to improved management and translate into lower mortality and less end-stage renal disease, improving both length and quality of life for people with CKD and diabetes.
Suggestions for audits to evaluate the implementation of the KDIGO guideline’s recommendations and practice points in primary care are summarised, along with their rationale, in Table 4.
Table 4: Audits to Optimise CKD Identification and Management in Primary Care
| Audit | Reason |
|---|---|
| People with diabetes with no recent ACR (>18 months) | To improve the diagnosis of albuminuria and CKD; permits prompt management of CKD and associated increased CVD risk |
| eGFR <60 ml/min/1.73 m2 on two measurements or albuminuria for >3 months not coded as CKD | Coding increases likelihood of management and raises awareness of the need for monitoring and drug dose alterations |
| Diabetes, hypertension, and albuminuria, no ACEI or ARB | To slow CKD progression |
| Type 2 diabetes and CKD and not on an SGLT2i (or long-acting GLP-1 RA if unsuitable for an SGLT2i) | To slow CKD progression and reduce CVD risk |
| CKD=chronic kidney disease; ACR=albumin:creatinine ratio; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; SGLT2i=sodium–glucose co-transporter-2 inhibitor; GLP-1 RA=glucagon-like peptide-1 receptor agonist. | |
Summary
The KDIGO guideline provides all audiences with evidence-based recommendations and practice points to guide the management of people with diabetes and CKD. It covers comprehensive care, glycaemic monitoring and targets, lifestyle interventions, antihyperglycaemic therapies, and self-management education. Where there is new evidence to support changes to the management of those with CKD and type 1 diabetes this is included, but in other areas the guideline focuses on type 2 diabetes and CKD. It is useful to compare this guideline with other guidance in common use. In the area of diabetes and CKD, the KDIGO guideline recommendations and guidance is broadly similar to other guidelines. All recommend SGLT2is for slowing progression of CKD and improving CVD outcomes in people with CKD and type 2 diabetes, but KDIGO differs in that it recommends first-line dual therapy with metformin and a SGLT2i provided that eGFR ≥30 ml/min/1.73 m2.
Dr Pam Brown
GP with an interest in diabetes, obesity, and lifestyle medicine, Swansea
Editor-in-Chief, Diabetes & Primary Care
Tutor, University of Warwick/iHeed Diabetes Diploma
Dr Colin Kenny
Diploma in Diabetes course leader, University of Warwick
| Implementation Actions for STPs and ICSs |
|---|
Written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved with implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system; KDIGO=Kidney Disease: Improving Global Outcomes; CKD=chronic kidney disease |
| COVID-19 Considerations |
|---|
CKD=chronic kidney disease; ACR=albumin:creatinine ratio; BP=blood pressure; HCP=healthcare professional; SGLT2i=sodium–glucose co-transporter-2 inhibitor; ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; DKA=diabetic ketoacidosis; CVD=cardiovascular disease |

