Dr Frances Akor and Professor Terry McCormack Discuss the Updated NICE Guideline on VTE, Focusing on Recommendations that are Relevant to Primary Care
| Read This Article to Learn More About: |
|---|
 
COVID-19 Considerations and Implementation Actions for STPs and ICSs are at the bottom of the article |
The term venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). Failure to diagnose and treat VTE promptly can result in fatal PE. Although advances have occurred in the diagnosis and management of acute VTE, it remains an important cause of morbidity and mortality. The NHS Outcomes Framework indicator for hospital-associated thrombosis (HAT) covering the period 2018/19 suggests a rate of death attributed to HAT of 57 per 100,000 adult hospital admissions, equating to thousands of deaths.1 Because of its wide variation in presentation, PE is frequently missed—autopsy studies suggest that PE was suspected in less than half of fatal cases.2
NICE Guideline (NG) 158 on Venous thromboembolic diseases: diagnosis, management and thrombophilia testing, published in March 2020, updates and replaces NICE Clinical Guideline 144 (published in 2012, last updated 2015). It covers adults with suspected or confirmed DVT or PE. It does not cover children or young people aged under 18 years, or pregnant women.3
This article outlines important changes to the guideline recommendations with a focus on those that are of particular relevance to primary care. The article does not cover the recommendations about thrombophilia testing. Since the publication of the original guideline, direct-acting oral anticoagulants (DOACs), such as apixaban and rivaroxaban, have become an established part of the oral anticoagulation landscape for the management of VTE. All four licensed DOACs are recommended for the acute treatment and secondary prevention of VTE through the NICE Single Technology Appraisal (STA) process.4–8
NICE has produced three helpful visual summaries covering the diagnostic pathways for DVT and PE and recommendations on the use of anticoagulation (see Figures 1–3). In addition, a useful resource impact report has been developed to support considerations around cost pressures and savings in the implementation of NG158.9
Diagnosis and Initial Management of VTE
The diagnostic pathway in NG158 includes new recommendations on the use of point-of-care and age-adjusted D-dimer tests and the use of the PE rule-out criteria (PERC). In terms of management, the key change is the recommendation to use DOACs in most cases, including in people with cancer.3
Please note that not all of the treatments discussed in this article currently (May 2020) have UK marketing authorisation for the indications mentioned; see notes to the recommendations in NG158.3 The prescriber should follow relevant professional guidance, taking full responsibility for all clinical decisions. Informed consent should be obtained and documented. See the General Medical Council’s guidance on Good practice in prescribing and managing medicines and devices10 for further information.
Diagnostic Pathway for DVT
For people presenting with signs or symptoms of DVT, the guideline continues to recommend an assessment of their general medical history followed by a physical examination to exclude other causes (see Figure 1).3 NICE continues to recommend the 2-level DVT Wells score to estimate the clinical probability of DVT when an event has not been ruled out by general medical history and physical examination.3 A DVT Wells score of ≥2 is predictive of DVT and termed ‘DVT likely’.3 Such patients should be offered a proximal leg vein compression ultrasound scan (CUS) with the results available within 4 hours if possible—steps to take when this is not possible are outlined in the next section of this article.3
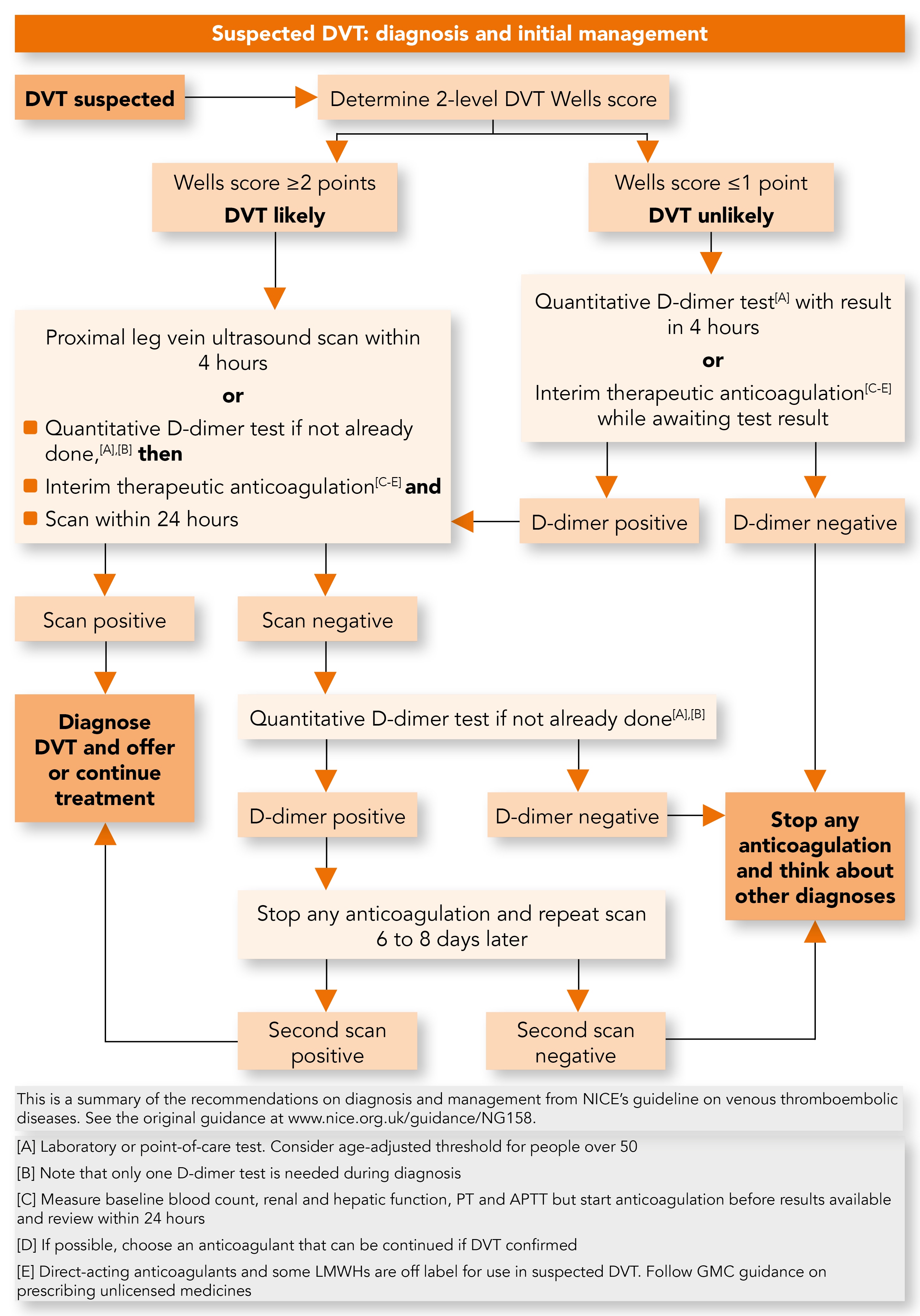
DVT=deep vein thrombosis; PT=prothrombin time; APTT=activated partial thromboplastin time; LMWHs=low molecular weight heparins
© NICE 2020. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available from: www.nice.org.uk/guidance/ng158 All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. See www.nice.org.uk/re-using-our-content/uk-open-content-licence for further details.
D-dimer Testing
Raised D-dimer levels are seen in a number of conditions other than VTE, including postoperatively, or with infection, cancer, inflammation, or trauma;11–13 therefore a raised D-dimer level alone is not predictive of VTE. The role of D-dimer testing is to identify those patients where VTE can be ruled out as a diagnosis as the test has a high negative predictive value.
A D-dimer should only be requested:3
- when the clinical probability of a DVT or PE is deemed ‘unlikely’ following use of the appropriate 2-level Wells score
- for patients with ‘likely’ DVT when:
- diagnostic imaging results will not be available within 4 hours
- the initial proximal CUS has not identified DVT, in order to ascertain whether repeat imaging should be done 6–8 days later.
When a CUS cannot be performed within 4 hours, a D-dimer test should be requested and after the test has been done, interim anticoagulation with either a DOAC or a parenteral anticoagulant commenced, unless contraindicated.3 In this scenario, the recommendation is that scan results should be available no later than 24 hours from request. The D-dimer test should be performed before commencing anticoagulation as anticoagulants can affect the results of the test.3
If a re-scan is indicated due to a positive D-dimer, then stopping anticoagulation will improve the likelihood of identifying a DVT that will extend proximally and require anticoagulation treatment. When the proximal CUS and the subsequent D-dimer are both negative, anticoagulation should be stopped and alternative diagnoses should be sought. To ensure that the ordering of D-dimer does not result in undue delay to the DVT diagnostic pathway, NICE now specifies a turnaround time of 4 hours for D-dimer test results. When this is not possible, interim anticoagulation should be initiated unless contraindicated.3
Of particular relevance to primary care, NG158 now states that the use of fully quantitative point of care tests (POCT) for D-dimer should be considered when laboratory facilities are not immediately available and that an age-adjusted D-dimer test threshold should be considered for people aged over 50 years. This approach optimises the timeliness of the diagnostic pathway, improves the accuracy of the D-dimer tests, reduces referrals for imaging, and reduces the need for interim anticoagulation.3 While some practices will need to purchase D-dimer POCT for the first time, there will be a requirement for other practices to switch from qualitative and semi-quantitative D-dimer tests to the more accurate quantitative tests.3 The authors suggest that one approach to implementing these NICE recommendations in a cost-efficient manner across a federation could be to arrange the diagnostic pathway so that nominated practices purchase the POCT for use within a federation hub-and-spoke model.
Diagnostic and Management Pathway for PE
For people presenting with signs or symptoms of PE (e.g. chest pain, shortness of breath, or coughing up blood), NICE recommends an assessment of their general medical history followed by a physical examination and chest X-ray to exclude other causes (see Figure 2).3 A brand-new recommendation from NICE here is to consider the use of the pulmonary embolism rule-out criteria (PERC)3,14 when the clinical suspicion of PE is low, to indicate whether there should be any further investigation before completely excluding PE as a cause.3
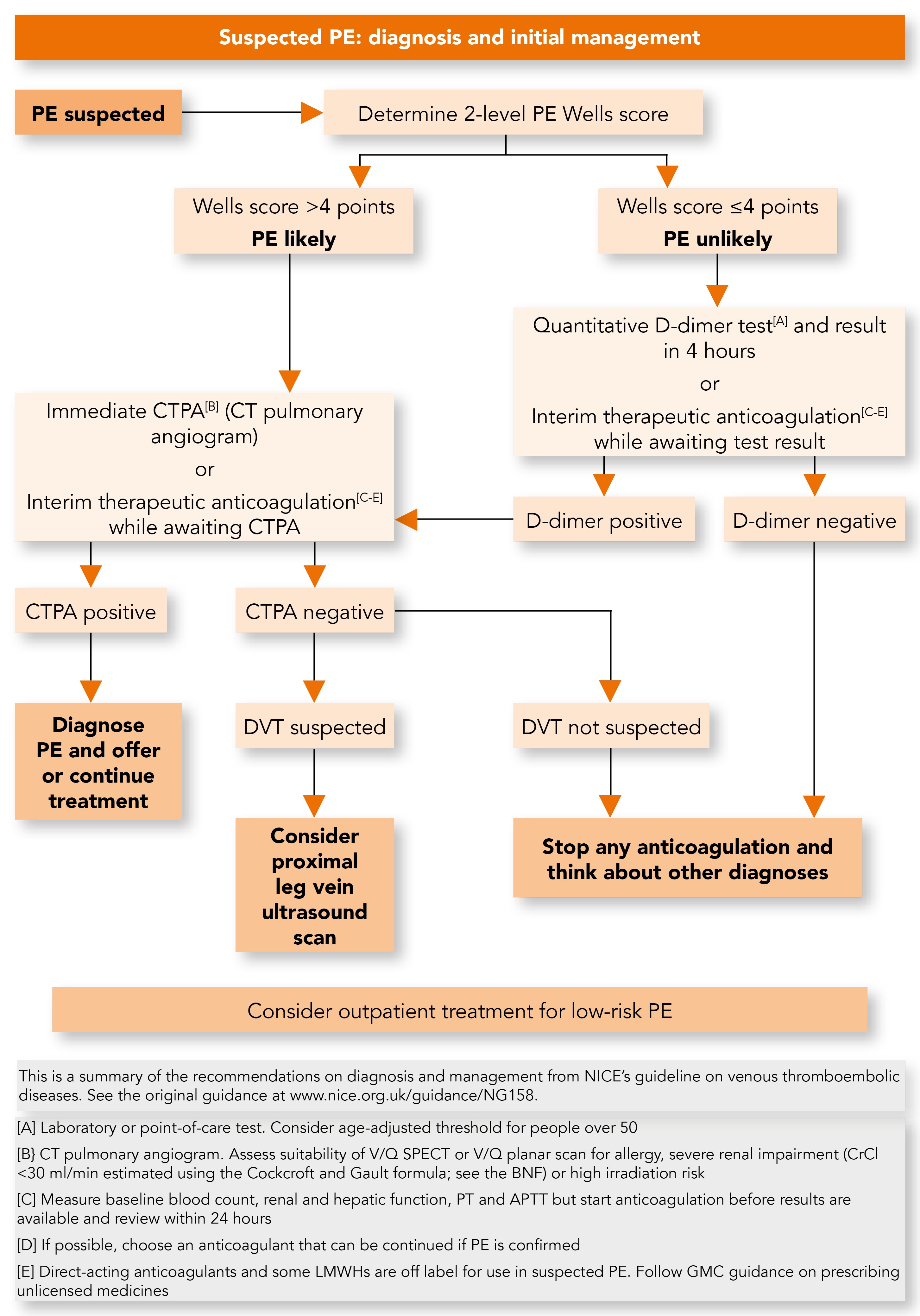
PE=pulmonary embolism; CT=computed tomography; DVT=deep vein thrombosis; V/Q SPECT=ventilation/perfusion single photon emission computed tomography; BNF=British National Formulary; PT=prothrombin time; APTT=activated partial thromboplastin time; LMWHs=low molecular weight heparins; GMC=General Medical Council
© NICE 2020. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available from: www.nice.org.uk/guidance/ng158 All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. See www.nice.org.uk/re-using-our-content/uk-open-content-licence for further details.
Review of the available evidence demonstrated that PERC can accurately eliminate PE as a possible diagnosis. However, as the evidence was limited, the recommendation is that PERC be considered rather than mandated as part of the initial assessment.3 It is hoped that increased use of PERC will reduce patient anxiety as well as reduce the need for D-dimer testing and imaging for people with none of the criteria for PE, leading to improvements in the PE pathway with reduced waiting times and use of anticoagulation.3
When PE is still suspected, NICE continues to recommend the 2-level PE Wells score to estimate the clinical probability of PE. A score of >4 is predictive of PE, i.e. ‘PE likely’.3 Such patients should be offered a computed tomography pulmonary angiogram (CTPA) immediately. When CTPA is not suitable (e.g. creatinine clearance <30 ml/min, contrast allergy, high risk from irradiation) then ventilation/perfusion single photon emission computed tomography (V/Q SPECT) scan if available, or V/Q planar scan, should be offered.3
The primary treatment for PE is anticoagulation, which must be started as soon as possible. NICE recommends that when immediate imaging is not possible interim anticoagulation must be commenced, provided there are no contraindications.3
When imaging results for PE are negative, a proximal leg vein CUS should be considered if a DVT is suspected. When DVT is not suspected, any interim anticoagulation should be stopped and alternative diagnoses should be sought. It should be explained to the patient that it is unlikely that they have a PE; they should also be educated about the signs and symptoms of VTE and when and where to seek further medical help.3
Outpatient Management of Low-risk PE
Outpatient management of low-risk PE is now common practice in settings such as ambulatory care units. NG158 recommends considering outpatient treatment for suspected or confirmed low-risk PE; a validated risk stratification tool should be used (ones in common use include the Pulmonary Embolism Severity Index (PESI)15 or simplified PESI16) to determine the suitability of outpatient treatment. In practice this is a two-step process; the risk stratification tools are first used to assess the prognostic risk associated with a PE event; when the score is sufficiently low against a validated tool the PE event is considered to be ‘low-risk’ such that outpatient management can then be reasonably considered. This recommendation is in line with 2018 British Thoracic Society guidance on the initial outpatient management of PE.17 Although the evidence comparing outpatient and inpatient management of low-risk PE is limited (which is why NG158 uses a lower strength ‘consider’ rather than ‘offer’ recommendation here), no evidence showed that outpatient treatment is less effective or less safe than inpatient treatment for people with low-risk PE. Outpatient care offers significant benefits both for people with PE and for hospital services.3
Information for People Having Outpatient Treatment
As part of outpatient management, NICE recommends that a plan for monitoring and follow-up (e.g. appointments) be agreed with people having outpatient treatment for suspected or confirmed low-risk PE. NICE recommends giving them:3
- written information about signs and symptoms of VTE and complications of VTE and treatment
- direct contact details of a healthcare professional or team with expertise in thrombosis who can discuss any new symptoms or signs, or other concerns
- information about out-of-hours services they can contact when their healthcare team is not available.
Anticoagulation
In the absence of contraindications, confirmed VTE requires anticoagulation for at least 3 months; in patients with active cancer NICE recommends anticoagulation for 3–6 months.3 NG158 defines ‘active cancer’ as: ‘Receiving active antimitotic treatment; or diagnosed within the past 6 months; or recurrent or metastatic; or inoperable. Excludes squamous skin cancer and basal cell carcinoma.’3
The NICE review of the clinical and cost-effectiveness of the DOACs, compared with low molecular weight heparin (LMWH) in combination with vitamin K antagonist anticoagulants (VKAs), favoured the use of apixaban and rivaroxaban for acute treatment in the first 3 months in most cases, with a strong ‘offer’ recommendation after taking into account co-morbidities, contraindications, and the person’s preferences. If neither apixaban nor rivaroxaban is suitable the alternatives that can be offered are:
- LMWH for at least 5 days followed by dabigatran or edoxaban or
- LMWH concurrently with a VKA for at least 5 days, or until the INR is at least 2.0 in two consecutive readings, followed by a VKA on its own.
The guideline also includes separate recommendations on anticoagulation for particular patient groups; these are outlined later in this article.
A weaker recommendation (1.4.8) suggests that practitioners ‘consider’ apixaban in the secondary prevention of unprovoked VTE. The preference for apixaban resulted from some evidence of the favourable bleeding profile of apixaban compared with rivaroxaban for acute treatment and in secondary prevention; however, the committee were not entirely convinced by this evidence as there were too few major bleeding episodes in the trials for them to be confident about the results. Rivaroxaban was marginally less cost effective than apixaban in the acute treatment setting.3
The licensing for dabigatran and edoxaban specifies initial treatment with parenteral anticoagulation for at least 5 days before they are started,5,6 making them less attractive and less suitable in the ambulatory care setting and more costly than their oral-only counterparts. However, cost-effectiveness is different from budget impact and different localities may benefit from a variety of procurement arrangements with manufacturers.
Prescribing Considerations
It is important to note that the DOAC oral-only regimens comprise higher initiation doses, which at a specified time point are reduced to the maintenance dose for the remainder of the 3-month treatment course (see the summary of product characteristics [SPC] for individual drugs for full details). Systems must be in place to ensure that patients change dose at the appropriate time and that prescribing and administration errors are avoided so that patients do not receive the wrong dose of medicine. Information about dose changes, adherence to medicine, management of inadvertent overdosing, and actions in the event of missed doses should be covered as part of patient education. In addition, pathways can be designed with follow up at critical time points to ensure that aspects such as dose changes are safely implemented.
NICE advises that interim anticoagulation should, if possible, be commenced with an agent that can be continued if VTE is confirmed, again favouring the use of oral-only DOACs over LMWH preceding DOAC or in combination with VKA.3 Before oral anticoagulation is started, baseline blood tests should be taken but treatment should not be delayed while results are awaited; instead, results should be reviewed and acted upon within 24 hours as necessary.3
In addition, it is important to ensure that a recent body weight measurement is available to support accurate calculation of renal function and appropriate dose selection. The trials of DOACs, their SPCs, and the British National Formulary use creatinine clearance (CrCl) calculated using the Cockcroft and Gault equation rather than estimated glomerular filtration rate (eGFR), which is reported by most pathology services as a measure of renal function.
Anticoagulation for VTE in Particular Patient Groups
The guideline makes separate recommendations on the use of anticoagulation for VTE in: renal impairment, people with cancer, antiphospholipid syndrome, and in people at extremes of weight (see Figure 3).
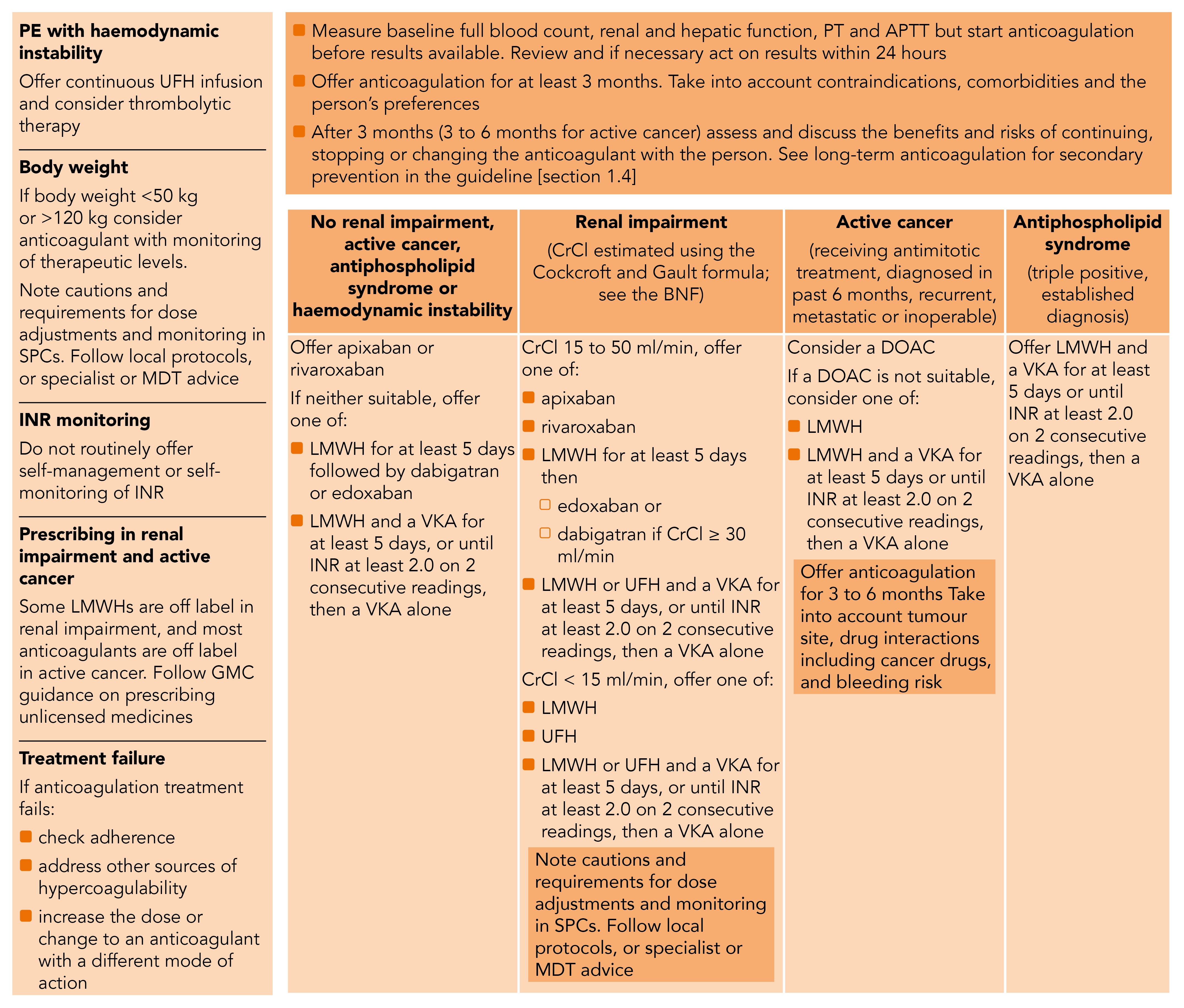
UFH=unfractionated heparin; SPCs=summary of product characteristics; MDT=multidisciplinary team; INR=international normalised ratio; LMWHs=low molecular weight heparins; GMC=General Medical Council; PT=prothrombin time; APTT=activated partial thromboplastin time; VKA=vitamin K antagonist anticoagulant; DOAC=direct-acting oral anticoagulant; CrCl=creatinine clearance; UFH=unfractionated heparin
© NICE 2020. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available from: www.nice.org.uk/guidance/ng158 All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. See www.nice.org.uk/re-using-our-content/uk-open-content-licence for further details.
Renal Impairment
Renal impairment can result in accumulation of anticoagulant agents, exposing patients to even greater risk of bleeding. Both apixaban and rivaroxaban can be used in renal impairment down to CrCl 15 ml/min and remain options in this patient group (Figure 3).3 Following at least 5 days of LMWH, edoxaban is also an option, while dabigatran (after LMWH) is not an option for people with more severe renal impairment (estimated CrCl 15 ml/min to 29 ml/min), as stated in its SPC.18,19 LMWH or unfractionated heparin (UFH) with VKA is also an acceptable option. As well as ensuring CrCl is calculated using up-to-date data it is also important to ensure that the appropriate dose is selected based on parameters including renal function, age, and drug interactions, following guidance in the relevant SPCs. There is evidence that suggests a considerable proportion of patients are receiving less than the SPC-recommended doses of DOACs;20 this may expose patients to excess risk of a VTE recurrence.
Active Cancer
One of the most prominent new recommendations is in the use of anticoagulation in patients with active cancer (see NICE’s definition, above). LMWHs are the only licensed anticoagulants for use in active cancer and have traditionally been the anticoagulant of choice in this patient group. However more recent, albeit relatively small, published studies have explored the use of rivaroxaban21 and edoxaban22 in patients with cancer and demonstrated non-inferiority to LMWH with respect to VTE recurrence (numerically lower recurrences) but higher rates of bleeding (particularly gastrointestinal and genitourinary bleeding, mainly in patients with gastrointestinal malignancies).3
Taking the comparative clinical efficacy and safety of DOACs together with their considerably lower cost compared with LMWH, DOACs were found to be substantially more cost-effective in patients with active cancer than LMWH.3 However, given the need for special consideration as to the appropriateness of DOACs for different cancer types and their possible interactions with cancer therapies, as well as the current lack of licensed indication for prescribing DOACs in active cancer, NICE’s recommendation is to ‘consider’ DOACs as first line rather than to ‘offer’ them.3
When DOACs are not considered appropriate, then LMWH alone or LMWH with a VKA are alternatives.3
The increased use of DOACs for patients with active cancer will conserve NHS resources, reduce injection burden for patients, and hopefully improve patient experience of anticoagulation treatment.
Antiphospholipid Syndrome
In June 2019, the Medicines and Healthcare products Regulatory Agency (MHRA) published a safety alert warning of an increase in VTE recurrence in people diagnosed with triple positive antiphospholipid syndrome taking a DOAC compared with those taking LMWH with VKA such as warfarin. Although people with antiphospholipid syndrome were not included in the guideline evidence review, NG158 reflects the importance of this alert by recommending that people with confirmed VTE and an established diagnosis of triple positive antiphospholipid syndrome are offered LMWH with VKA.3
People at Extremes of Weight
Due to the influence of body weight on the absorption, distribution and elimination of anticoagulants, NICE recommends that consideration should be given to regular monitoring of anticoagulation levels for people with confirmed VTE who weigh less than 50 kg or more than 120 kg to ensure therapeutic anticoagulation.3
Risks and Benefits of Long-term Anticoagulation
Traditionally, provoked VTE, where the provoking risk factor is no longer present and the clinical course has been uncomplicated, is treated for at least 3 months and the updated NICE guideline still recommends that consideration should be given to stopping anticoagulation after 3 months in this patient group and after 3–6 months in patients with active cancer. When anticoagulation is stopped, patients must be given information about the risk of having another VTE as well as the information outlined under heading ‘Information for people having outpatient treatment’, above.3
For patients with unprovoked VTE, consideration should be given to continuing anticoagulation beyond 3 months (beyond 6 months in patients with active cancer). Factors that should be considered when making a decision about whether to continue anticoagulation include the balance between the person’s risk of VTE recurrence and their risk of bleeding. The risks and benefits of long-term anticoagulation should be discussed with the person, and their preferences taken into account.3 DOACs have a more favourable bleeding profile than VKAs such as warfarin; therefore in most individuals with unprovoked VTE and low bleeding risk, the benefit of continuing anticoagulation now outweighs the risk of a major bleed and NICE recommends that this be explained to people falling within this category.
NICE recommends that a discussion about stopping anticoagulation should take place with people who have unprovoked VTE and a HAS-BLED23 score of 4 or more, that cannot be modified.3 For people who decline long-term anticoagulation where the benefits of continued therapy outweigh the risks, the use of aspirin 75 mg or 150 mg daily should be considered.3
A review of general health, risk of VTE recurrence, bleeding risk, and treatment preferences should be undertaken at least once a year for patients receiving long-term anticoagulation or aspirin therapy for secondary prevention of VTE.3
The 2012 guideline controversially suggested that people with unprovoked VTE undergo screening for cancer, including mammograms and CT imaging. The updated guideline recommends a review of the medical history and baseline blood tests, and a full physical examination only. Now, any further investigations should be offered only if patients have relevant clinical symptoms or signs (see NG12 on suspected cancer24). This recommendation will not only reduce costs and imaging appointments but also alleviate the anxiety of patients who would have previously been needlessly referred for imaging.3
Treatment Failures
In treatment failures the guideline recommends checking adherence to anticoagulation treatment, addressing other potential sources of hypercoagulability, increasing the dose of anticoagulant, or switching to an anticoagulant with a different mode of action.3
When Anticoagulation is Contraindicated
Due to the limited evidence of benefit, the updated guideline recommends that inferior vena cava (IVC) filters should only be used in the context of a clinical trial, or when anticoagulation is contraindicated, or when a PE has occurred despite adequate anticoagulation. Before the IVC filter is fitted, there must be a clear plan in place for removing it at the earliest possible opportunity.3
Summary
NICE guideline 158 represents an opportunity for primary care to be more involved in a number of aspects of the management of VTE, including low-risk PE and VTE in patients with active cancer. Moving VTE services out of secondary care into primary care is expected to improve the patient experience and deliver cost savings.
To implement NICE recommendations successfully in primary care, some localities will require pathway redesign to ensure straightforward referral mechanisms as well as availability of slots for imaging scans in their local service, with results available within NICE recommended timeframes. In addition, some will need to invest in quantitative point-of-care D-dimer tests to optimise the timeliness of the pathway. It will be important to agree and clearly define the pathway across primary and secondary care, including who has clinical responsibility for the patient at different stages of the pathway and in different scenarios, to ensure a safe and timely patient journey.
Dr Frances Akor
Consultant Pharmacist (Anticoagulation), Imperial College Healthcare NHS Trust
Member of the guideline development group for NG158
Professor Terry McCormack
Vice-President British and Irish Hypertension Society
Honorary Professor of Primary Care Cardiovascular Medicine, Hull York Medical School
Member of the guideline development group for NG158
| COVID-19 Considerations |
|---|
These are the views of the authors and not the NICE VTE Guideline Committee. VTE=venous thromboembolism; DVT=deep vein thrombosis; PERC=pulmonary embolism rule-out criteria; DOACs=direct-acting oral anticoagulants; INR=international normalised ratio; PE=pulmonary embolism; LMWHs=low molecular weight heparins |
| Implementation actions for STPs and ICSs |
|---|
written by Dr David Jenner, GP, Cullompton, Devon The following implementation actions are designed to support STPs and ICSs with the challenges involved with implementing new guidance at a system level. Our aim is to help you consider how to deliver improvements to healthcare within the available resources.
STP=sustainability and transformation partnership; ICS=integrated care system; VTE=venous thromboembolism; PCNs=primary care networks; CCG=clinical commissioning group; DOACs=direct-acting oral anticoagulants; PERC=pulmonary embolism rule-out criteria |
The guideline referred to in this article was produced by Guideline Updates Team for the National Institute for Health and Care Excellence (NICE). The views expressed in this article are those of the authors and not necessarily those of NICE. National Institute for Health and Care Excellence (2020) Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available from www.nice.org.uk/guidance/ng158 |

